Olefin epoxidation method
An epoxidation and olefin technology, applied in organic chemistry and other directions, can solve the problems of high hydrogen peroxide conversion, inability to balance, and prolong product selectivity, so as to reduce costs, meet large-scale industrial production, and prolong single-pass reaction time. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0029] According to a preferred embodiment of the present invention, the feed mass hourly space velocity of the hydrogen peroxide is 0.05-0.3h -1 , preferably, the feed mass hourly space velocity of the hydrogen peroxide is from 0.05-0.15h -1 Increase to 0.1-0.3h -1 ; Further preferably, the feed mass hourly space velocity of the hydrogen peroxide is from 0.08-0.13h -1 Increase to 0.12-0.2h -1 .
[0030] The hydrogen peroxide can be a pure product, but it is preferably an aqueous solution of hydrogen peroxide from the viewpoint of economy and safety, and the mass percentage of hydrogen peroxide can be selected from 5%-90%, preferably 20%-60% %, such as 27.5%, 30%.
[0031] The method provided by the present invention is applicable to the epoxidation of various olefins, for example, the olefins may be selected from at least one of propylene, 3-chloropropene, 1-butene, 1-pentene and 1-hexene. Correspondingly, the epoxy olefin is selected from at least one of propylene oxide...
Embodiment 1
[0063] The shaped titanium-silicon molecular sieve catalyst is produced by Sinopec Catalyst Company according to the preparation method disclosed in Example 3 of CN201010184391.9, and the mass content of TS-1 in the shaped titanium-silicon molecular sieve catalyst is 60%. Carry out the epoxidation reaction of 3-chloropropene and hydrogen peroxide in a pilot-scale jacketed tubular fixed-bed reactor (catalyst bed height of 7.6 meters and a diameter of 24 millimeters) with an aspect ratio of 316.7, and the reaction feed In the process, the addition of ammonia is 0.036% of the mass of 3-chloropropene, and the mass content of hydrogen peroxide is 30.0%; the temperature of the liquid heat transfer medium water introduced in the reactor jacket is gradually increased from 35°C to 60°C, And the heating rate of water is gradually reduced from 0.0417°C / hour to 0.0083°C / hour, the flow rate of water is gradually increased from 0.15m / s to 0.5m / s, and the mass hourly space velocity of hydroge...
Embodiment 2
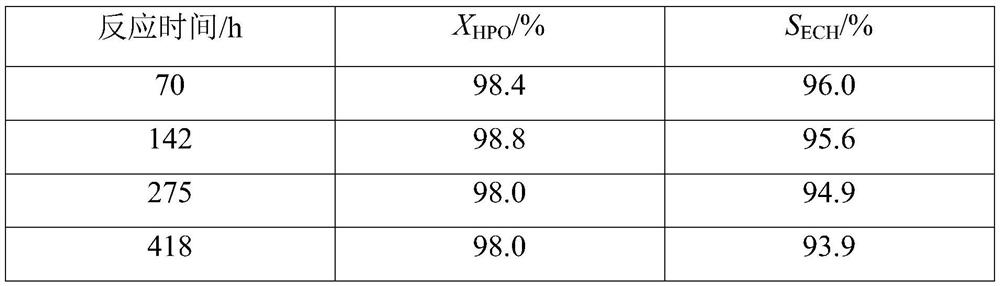
[0068] Adopt the same method as Example 1 to carry out the epoxidation reaction of 3-chloropropene and hydrogen peroxide, the difference is that in the pilot scale jacketed tubular fixed-bed reactor (catalyst bed height of 253.3) Carry out the epoxidation reaction of 3-chloropropene and hydrogen peroxide in 7.6 meters, diameter is 30 millimeters), the mass content of hydrogen peroxide is 27.5%; Gradually decrease from 0.0667°C / hour to 0.010°C / hour, the flow rate of water gradually increases from 0.2m / s to 1.0m / s, and the mass hourly space velocity of hydrogen peroxide feeds from 0.112h -1 Gradually increase to 0.141h -1 , and the results of the epoxidation reaction are shown in Table 2.
[0069] Table 2
[0070] Reaction time / h X HPO / %
[0071] Note: The average value of 1292h refers to the X of 216 sampling data between 1292h HPO and S ECH average of.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com