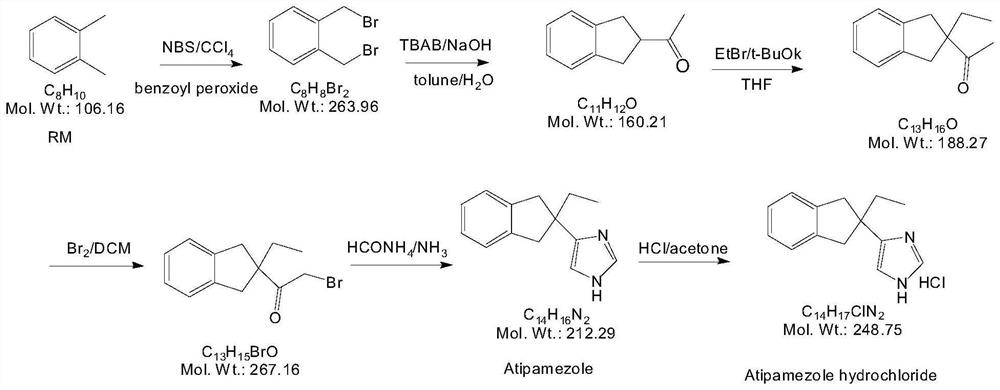
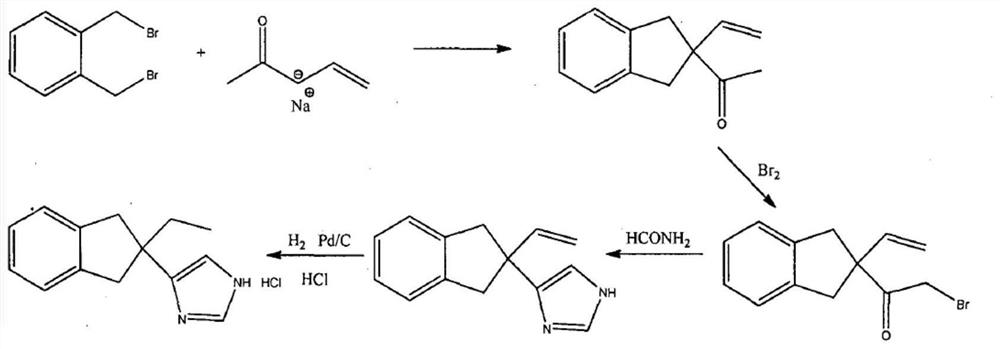
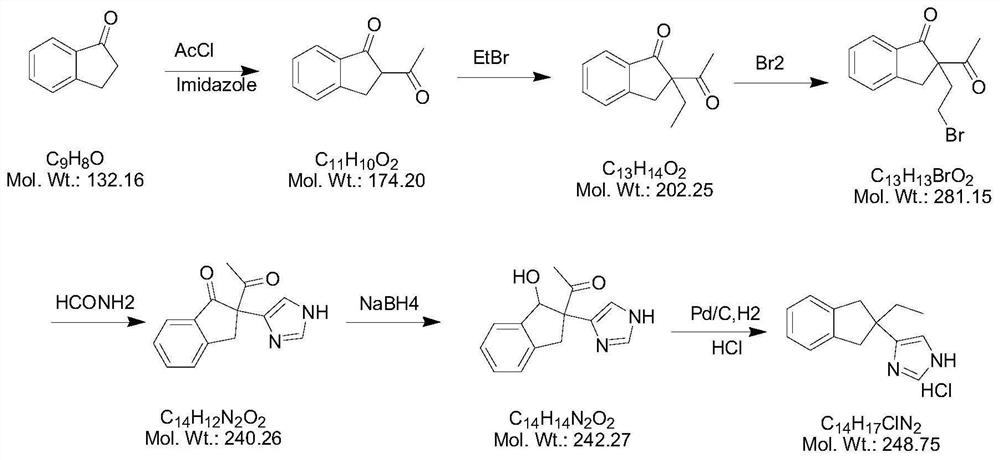
Preparation method of atipamezole
A technology of atipamezole and compounds, which is applied in the field of preparation of atipamezole, can solve the problems of harsh reaction conditions, inability to withstand high-pressure hydrochloric acid reaction, and high requirements for reaction equipment, so as to achieve high selectivity and avoid the formation of impurities , good product yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1 prepares compound 2
[0032] In a cold water bath at 0-5°C, take 7g of compound 1 and add 105ml of absolute ethanol (15V / W), stir, add 2.8g of sodium borohydride, react for 24 hours, spot the plate to monitor the reaction, and take acetone when compound 1 disappears 30ml was added dropwise to the reaction mixture to terminate the reaction. The solvent was removed under reduced pressure, 200 ml of dichloromethane was added, stirred for 2 hours, filtered, and the solvent was removed under reduced pressure to obtain 5.92 g of crude compound 2, yield: 84.57%.
Embodiment 2
[0033] Embodiment 2 prepares atipamezole
[0034] Take 5.4g of compound 2 and add it to 20ml of acetonitrile, then add 10ml of dichloromethane, cool down to -20~-10°C, add 20ml of iodotrimethylsilane to react, TLC detects that the reaction is complete, add 2M sodium thiosulfate to terminate the reaction, Stand still to separate the liquids, wash the organic phase once with saturated saline, concentrate the organic phase to remove the solvent, add 50ml of 2M hydrochloric acid, react at 100°C for 1-1.5 hours, remove the protecting group, filter, adjust the pH of the aqueous phase with 40% sodium hydroxide >10, filtered to obtain 2.3g of light yellow solid. Yield 92%.
Embodiment 3
[0035] Embodiment 3 prepares atipamezole hydrochloride
[0036] Take 4 grams of atipamezole, add two equivalents of ethanol hydrochloride (6M) dropwise in a cold water bath at 5°C, and stir for 30 minutes after the addition; remove the solvent under reduced pressure; then add 3-5 (v / w) anhydrous Slurry with ethanol for 3 hours at a temperature of 25-30°C and filter. The filter cake was dried under reduced pressure at 60°C for 24 hours to obtain atipamezole hydrochloride: 3.81 g, yield 95.25%.
[0037] High-performance liquid chromatography detection: the content is greater than 99%, and the single impurity content is less than 0.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com