Synthesis process of hydrocortisone hemisuccinate
A technology of hydrocortisone and succinate, applied in steroids, organic chemistry, etc., can solve the problem of triethylamine catalytic reaction activity, low selectivity, large amount of succinic anhydride and reaction solvent, pine hemisuccinic acid, etc. The problem of low ester yield is to achieve the effect of process optimization, simple post-processing operation and reduction of processing cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
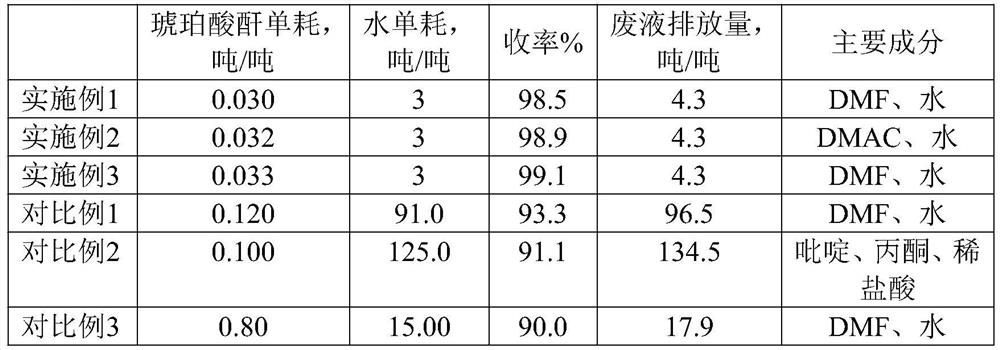
[0028] Put 130g of dimethylformamide (DMF), 100g of hydrocortisone, 30g of succinic anhydride, and 3g of DMAP into the reaction kettle, and stir at the same time; heat to dissolve, and keep it warm for 3 hours; cool down to 40°C, slowly Add 200g of water, and at the same time lower the temperature to below 10°C, heat and crystallize for 1 hour; centrifuge, filter and wash with water twice; dry for 6 hours at 100°C and a vacuum of 0.095MPa or more to obtain half of hydrocortisone 126.2 g of white crystalline solid of succinate, yield 98.9%.
Embodiment 2
[0030] Put 135g of dimethylacetamide (DMAC), 100g of hydrocortisone, 32g of succinic anhydride, and 6g of pyridine into the reaction kettle, and stir at the same time; heat and dissolve to 45°C, and keep the reaction for 6 hours; Slowly add 200g of water, and at the same time lower the temperature to below 10°C, heat and crystallize for 1.5 hours; centrifugally filter, and wash with water twice, put the washed crystals in a vacuum drying oven, at 105°C, vacuum degree above 0.095MPa , and dried for 6 hours to obtain 125.8 g of hydrocortisone hemisuccinate white crystalline solid with a yield of 98.6%.
Embodiment 3
[0032] Put 130g of dimethylformamide (DMF), 100g of hydrocortisone, 33g of succinic anhydride, and 4g of DMAP into the reaction kettle, and stir at the same time; heat to dissolve, and keep it warm for 4 hours; cool down to 40°C, slowly Add 200g of water, and at the same time lower the temperature to below 10°C, heat and crystallize for 1 hour; centrifuge, filter and wash with water twice; dry for 8 hours at 95°C and a vacuum of 0.095MPa or more to obtain half of hydrocortisone 126.8 g of white crystalline solid of succinate, yield 99.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com