Novel synthesis method of 2-hydroxy-5-nonylbenzaldoxime
A technology of nonylbenzaldehyde oxime and synthesis method, which is applied in the novel synthesis field of 2-hydroxy-5-nonylbenzaldehyde oxime, can solve the problems such as low conversion rate and purity, insufficient resource utilization, complicated process, etc. The effect of conversion rate and product purity, short synthesis process route and high synthesis efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
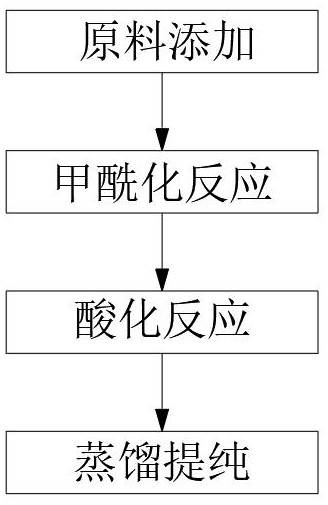
[0025] Embodiment 1, the present invention provides a kind of technical scheme, the novel synthetic method of 2-hydroxyl-5-nonylbenzaldehyde oxime, and this synthetic method comprises the following steps:
[0026] Step 1: Adding raw materials, respectively adding nonylphenol, toluene, and magnesium chloride into the reaction flask, the feeding molar ratio is 1:5.6:1.2, adding 76 g of triethylamine dropwise under stirring and gradually raising the temperature;
[0027] Step 2: Formylation reaction, weigh 52.5g of paraformaldehyde, add it to the reaction bottle in step 1 in batches, 3-4g each time, the interval is 5-10 minutes, and the process of adding paraformaldehyde is maintained at about 3 hours;
[0028] Step 3: Acidification reaction, lower the temperature of the reaction product after the formylation reaction in step 2 to 60°C, then slowly add 2.5N hydrochloric acid solution into the reaction flask in step 1, and keep the reaction at a temperature of 80°C 1 hour;
[00...
Embodiment 2
[0031] Embodiment two, the present invention provides a kind of technical scheme, the novel synthetic method of 2-hydroxyl-5-nonylbenzaldehyde oxime, and this synthetic method comprises the following steps:
[0032] Step 1: Adding raw materials, respectively adding nonylphenol, toluene, and magnesium chloride into the reaction flask, the feeding molar ratio is 1:5.6:1.2, adding 76 g of triethylamine dropwise under stirring and gradually raising the temperature;
[0033] Step 2: Formylation reaction, weigh 52.5g of paraformaldehyde, add it to the reaction bottle in step 1 in batches, 3-4g each time, the interval is 5-10 minutes, and the process of adding paraformaldehyde is maintained at about 3 hours;
[0034] Step 3: Acidification reaction, lower the temperature of the reaction product after the formylation reaction in step 2 to 60°C, then slowly add 2.5N hydrochloric acid solution into the reaction flask in step 1, and keep the reaction at 100°C 1 hour;
[0035] Step 4: Di...
Embodiment 3
[0037] Embodiment three, the present invention provides a kind of technical scheme, the novel synthetic method of 2-hydroxyl-5-nonylbenzaldehyde oxime, and this synthetic method comprises the following steps:
[0038] Step 1: Adding raw materials, respectively adding nonylphenol, toluene, and magnesium chloride into the reaction flask, the feeding molar ratio is 1:5.6:1.2, adding 76 g of triethylamine dropwise under stirring and gradually raising the temperature;
[0039] Step 2: Formylation reaction, weigh 52.5g of paraformaldehyde, add it to the reaction bottle in step 1 in batches, 3-4g each time, the interval is 5-10 minutes, and the process of adding paraformaldehyde is maintained at about 3 hours;
[0040] Step 3: Acidification reaction, lower the temperature of the reaction product after the formylation reaction in step 2 to 60°C, then slowly add 2.5N hydrochloric acid solution into the reaction flask in step 1, and keep the reaction at 60°C 1 hour;
[0041] Step 4: D...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com