Closed-loop recovery method of lithium-containing molten salt
A recycling method and molten salt technology, applied in chemical instruments and methods, inorganic chemistry, electrical components, etc., can solve the problems of excessive use of electrode materials, inability to achieve large-scale production and preparation, and waste of molten salt.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
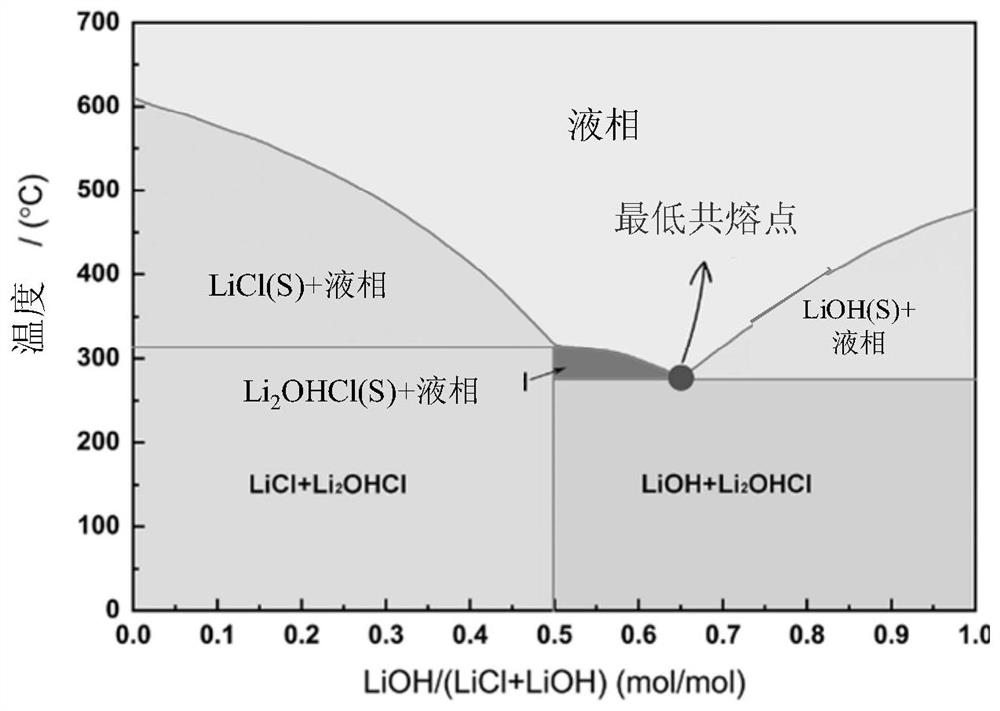
[0062] Configure the lithium-containing molten salt system, the lithium-containing molten salt system is a mixed system of the first lithium salt and the second lithium salt, the first lithium salt is LiCl, the second lithium salt is LiOH, the amount of LiOH substance is 86mmol, and the amount of LiCl substance The amount is 50.7mmol;
[0063] Will LiNi 0.5 co 0.2 mn 0.3 o 2 Precursor mixed with lithium-containing molten salt system, LiNi 0.5 co 0.2 mn 0.3 o 2 The molar ratio of the precursor to the lithium-containing molten salt system is 1:5, and the mixture is obtained. The mixture is transferred to a high-temperature kiln and fed with oxygen. First, it is preheated at 300°C for 2 hours, and then heated to 850°C. , keep the temperature for 6h for sintering, cool after sintering, wash with water three times, and collect the filtrate and sediment;
[0064] Introduce oxygen into the precipitate and heat it to 850°C, keep it warm for 2 hours to dry, and obtain the lithi...
Embodiment 2
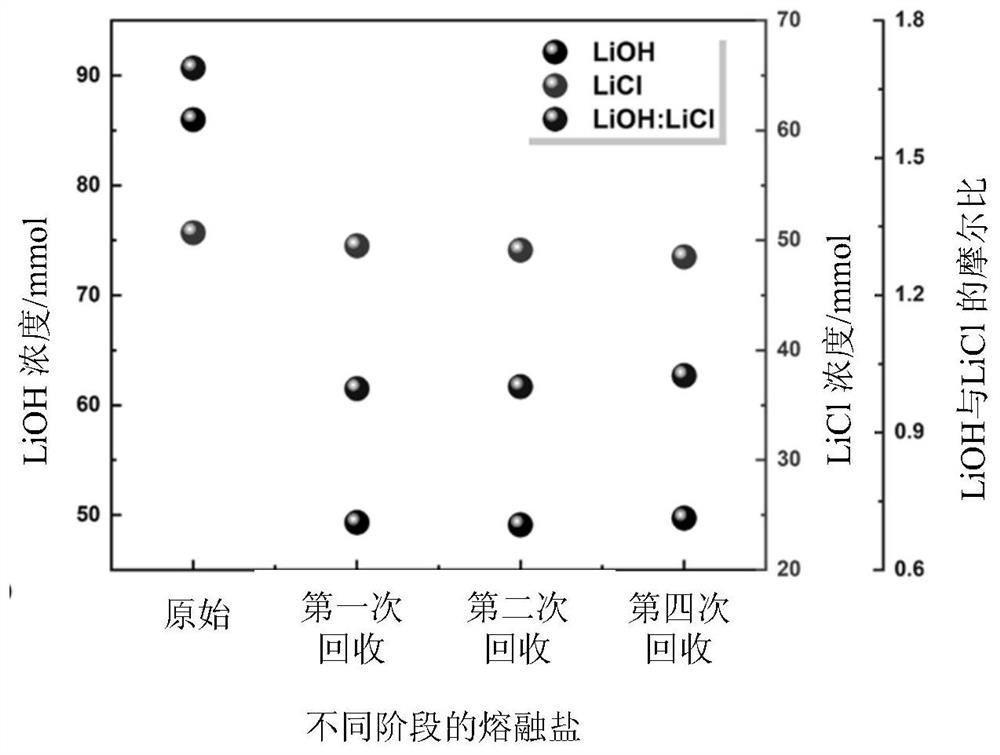
[0067] Titration analysis
[0068] Obtain after the filtrate of recovery is dried and reclaim lithium-containing molten salt, carry out titration test:
[0069] The titration of LiOH: take methyl red-bromocresol green as indicator, titrate the total alkalinity in the sample filtrate with a certain concentration of hydrochloric acid standard titration solution, calculate the content of lithium hydroxide with the amount of consumption of hydrochloric acid standard titration solution, 3 Group parallel.
[0070] Titration of LiCl: with standard AgNO 3 Solution titration of Cl in sample filtrate - Form AgCl precipitate, with potassium chromate as indicator, when Cl - After precipitation, the Ag + with CrO 4 2- Formation of red precipitate 2Ag + +CrO 4 2- =Ag 2 CrO 4 ↓(red) indicates the arrival of the end point. According to AgNO 3 The amount of (Ag needs to be deducted + The amount consumed by the hydroxide precipitation reaction with LiOH) can be calculated as Cl -...
Embodiment 3
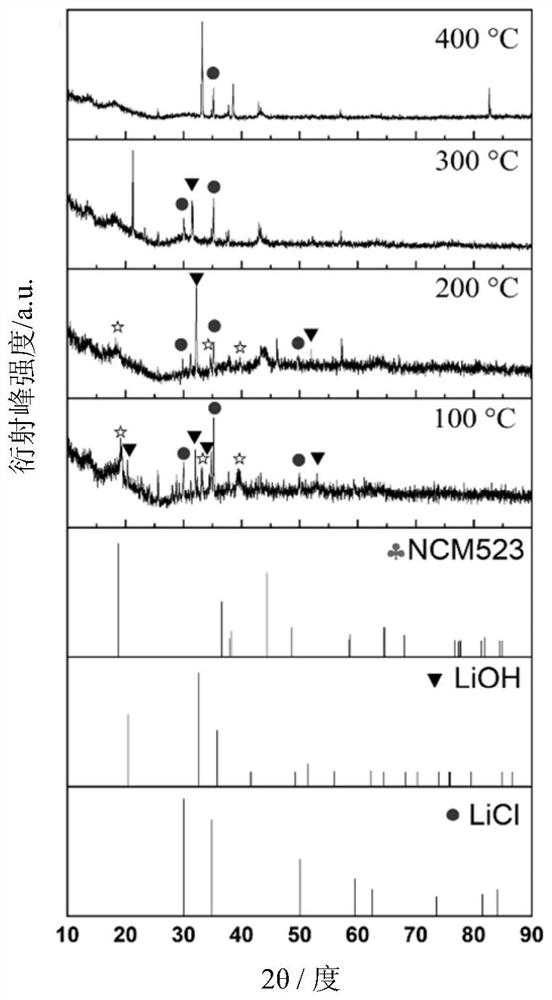
[0072] For the LiNi of Example 1 0.5 co 0.2 mn 0.3 o 2 The mixture obtained after the precursor was mixed with the lithium-containing molten salt system was tested by in-situ XRD.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap