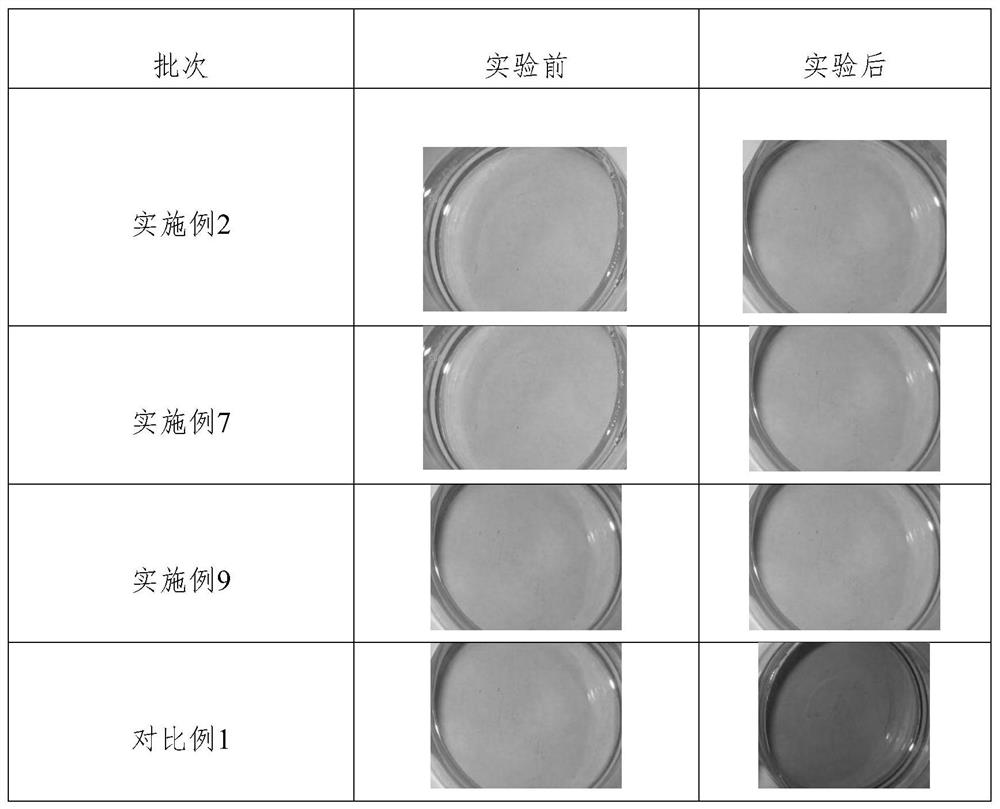
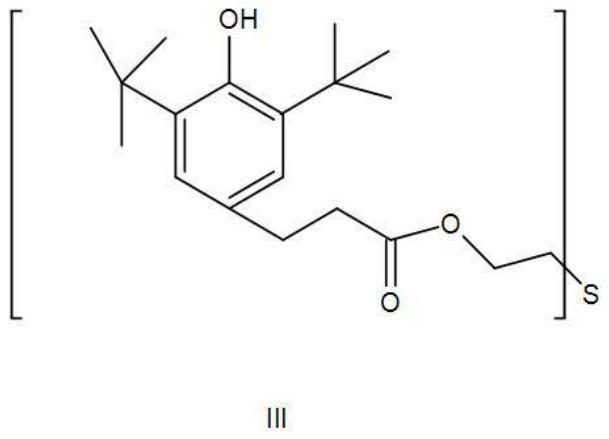
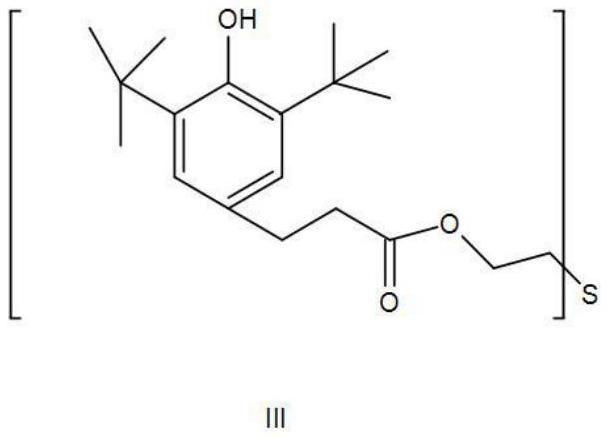
Synthesis method of thioether phenolic antioxidant
A technology of phenolic antioxidants and synthetic methods, which is applied in the direction of sulfide preparation, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problems of poor catalytic effect of catalysts, cumbersome catalyst treatment, and inability to recycle, etc., to achieve Good chromaticity difference at high temperature, no pollution, and the effect of improving product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A
[0054] Clean the silica carrier with deionized water, activate at 105°C, and purging with nitrogen for 2 hours, take 20g of silica and put it in 100ml of methanol solution with a concentration of 35% lithium acetate, at 20-25°C Immerse for 12 hours, filter, and dry at 100°C for 2 hours to obtain a supported transesterification catalyst silica / lithium acetate.
Embodiment B
[0056] Clean the hydrotalcite carrier with deionized water, activate it at 140°C, and purging with nitrogen for 3 hours, take 15g of hydrotalcite and put it into 150ml of methanol solution with a lithium methylate concentration of 20%, and immerse it at 32-40°C for 9h. Filter and dry at 120°C for 2 hours to obtain a supported transesterification catalyst hydrotalcite / lithium methoxide.
Embodiment C
[0058] Clean the HY molecular sieve carrier with deionized water, activate at 90°C, and purging with nitrogen for 3h, take 20g of HY molecular sieve and put it into 100ml of methanol solution with a concentration of 40% dioctyltin oxide, and immerse it at 40-50°C for 8h , filtered, and dried at 70°C for 4h to obtain a supported transesterification catalyst HY molecular sieve / dioctyltin oxide.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com