Organic room-temperature phosphorescent material as well as preparation method and application thereof
A room temperature phosphorescent and phosphorescent material technology, applied in the fields of luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve the problems of difficult information erasure, difficult to meet application requirements, unable to apply solid or gel materials, etc. Anti-counterfeiting and display scene requirements, low cost, strong machinability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
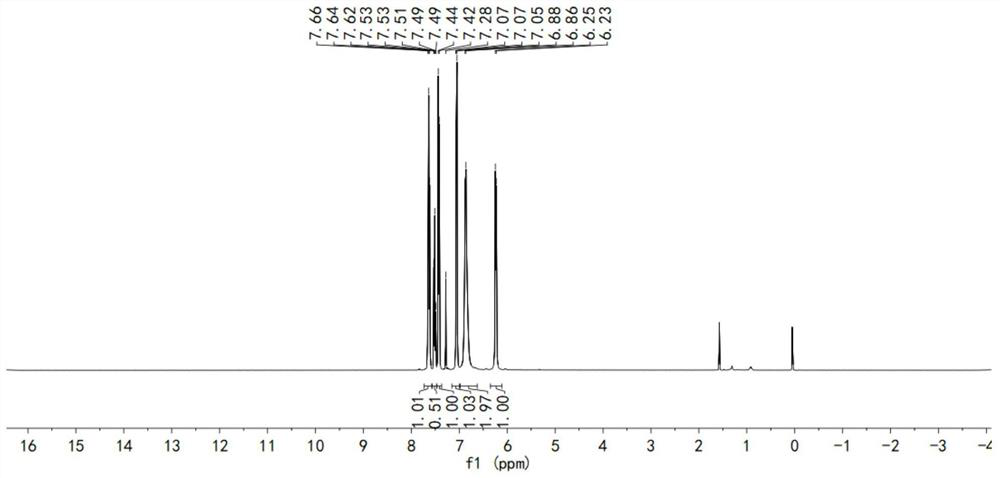
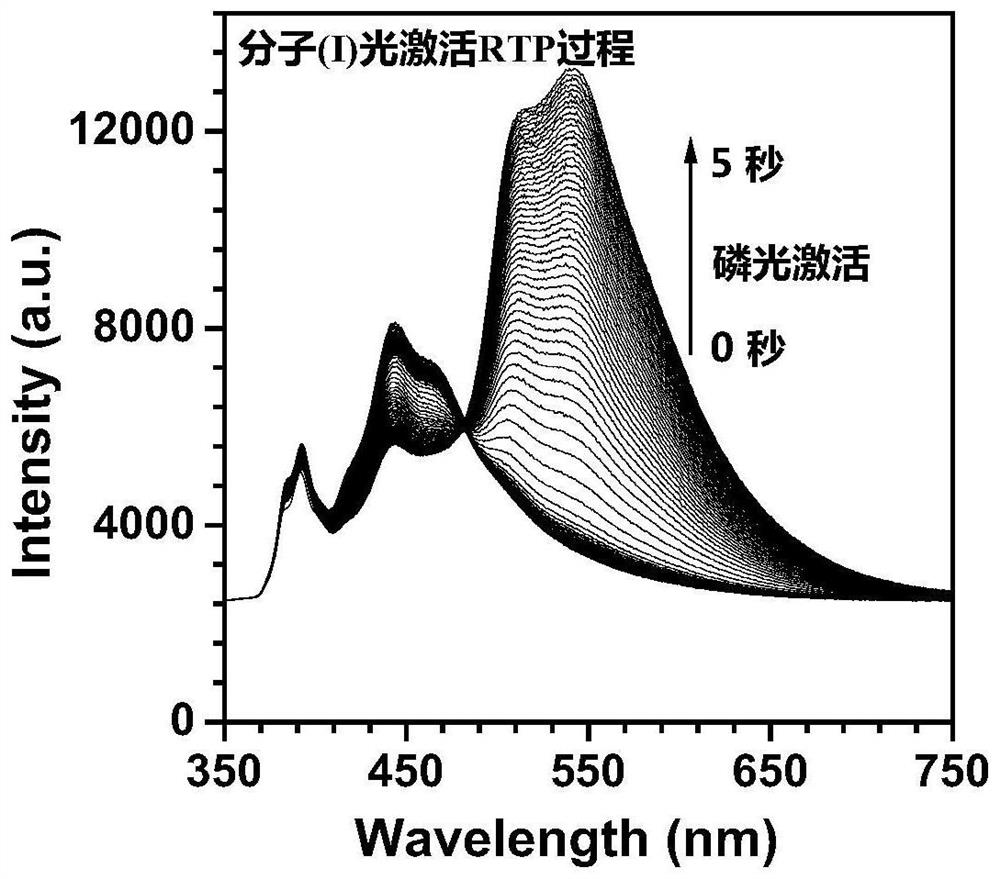
[0064] An organic room-temperature phosphorescent material, said phosphorescent material comprising any one of the phosphorescent materials represented by formula (I), formula (II), and formula (III);
[0065]
[0066] Further, formula (I) is obtained by coupling reaction of phenothiazine and monohalogenated benzene; formula (II) is obtained by coupling reaction of phenothiazine and p-substituted halogenated benzene; formula (III) is obtained by coupling reaction of phenothiazine and 1,3,5-substituted halogenated benzene coupling reaction.
[0067] in:
[0068] The structural formula of phenothiazine is (a);
[0069] The structural formula of monohalogenated benzene is (b);
[0070] The structural formula of p-substituted halobenzene is (c);
[0071] The structural formula of 1,3,5-substituted halobenzene is (d);
[0072] In the formula, R=Br, I; R 1 =Br,I;R 2 =Br,I;R 3 =Br,I.
[0073] Further, in formula (I), the ratio of the amount of phenothiazine to mono...
Embodiment 2
[0075] A preparation method of the organic room temperature phosphorescent material described in embodiment 1, comprising the following steps:
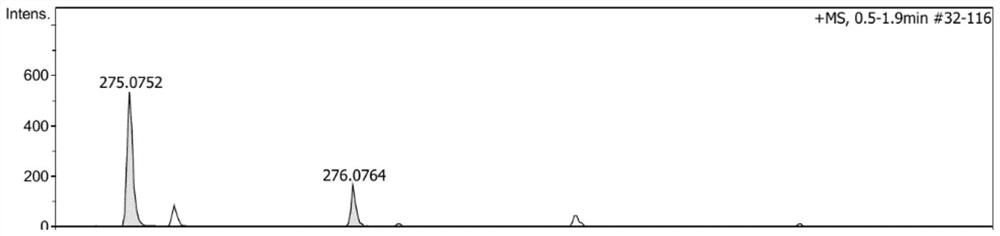
[0076] S1, under nitrogen protection state, get 1.99g (10mmol) phenothiazine, 2.04g (10mmol) iodobenzene, 1.68g (15mmol) potassium tert-butoxide (t-BuOK), 0.11g (0.5mmol) palladium acetate ( Pd(OAc) 2 ) and 0.25mL (0.25mmol) tri-tert-butylphosphine (P(t-Bu) 3 ) was dissolved in 90mL of toluene solution, heated to reflux for 24 hours. Cool to room temperature, heat and evaporate to remove the solvent, extract three times with dichloromethane, add anhydrous magnesium sulfate to the organic phase and dry. The crude product was obtained by filtration, and then purified by column chromatography to obtain the product of formula (I), the product quality was 2.6 g, and the yield was 94.5%. Concrete preparation reaction formula is:
[0077]
[0078] S2, get 1.0 mg of the formula (I) product obtained in S1 and 100 mg of polymethyl methac...
Embodiment 3
[0087] A preparation method of the organic room temperature phosphorescent material described in embodiment 1, comprising the following steps:
[0088] S1. Under nitrogen protection, take 3.98g (20mmol) phenothiazine, 2.81g (10mmol) p-bromoiodobenzene, 2.88g (30mmol) sodium tert-butoxide (t-BuONa), 0.11g (0.5mmol) acetic acid Palladium (Pd(OAc) 2 ) and 0.25mL (0.25mmol) tri-tert-butylphosphine (P(t-Bu) 3 ) was dissolved in 120mL of toluene solution, heated to reflux for 24 hours. Cool to room temperature, heat and evaporate to remove the solvent, extract three times with dichloromethane, add anhydrous magnesium sulfate to the organic phase and dry. The crude product was obtained by filtration, and then purified by column chromatography to obtain the product of formula (II), with a product quality of 3.8 g and a yield of 80.9%. Concrete preparation reaction formula is:
[0089]
[0090]S2, take 0.5 mg of the formula (I) product obtained in S1 and 100 mg of polymethyl met...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com