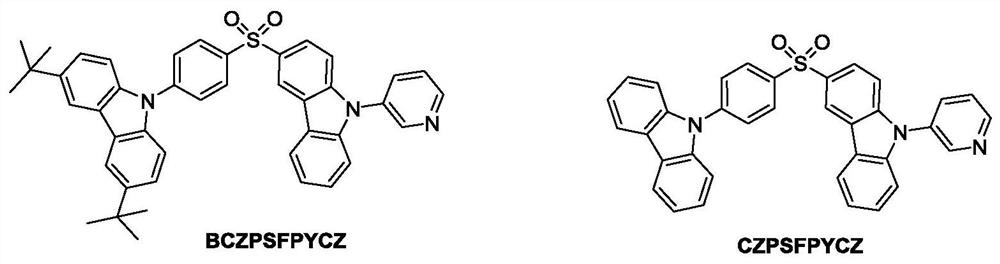
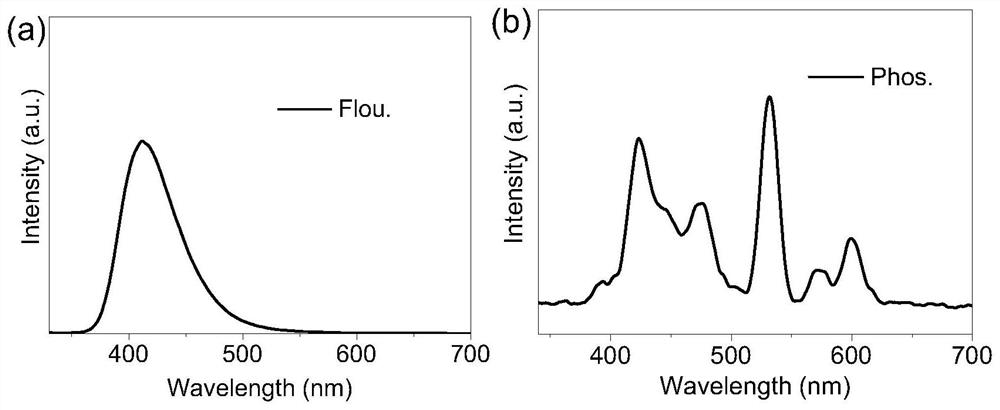
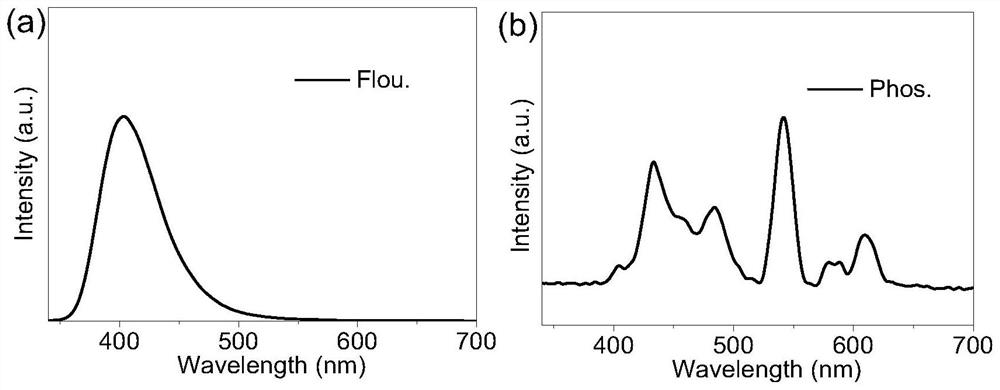
Multistimuli-responsive material as well as preparation method and application thereof
A stimulus-response and multi-stimulus technology, applied in luminescent materials, chemical instruments and methods, character and pattern recognition, etc., can solve problems such as the unclear relationship between molecular structure and luminescent mechanism, and achieve remarkable results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The preparation method of the stimulus response material BCZPSFPYCZ comprises the following steps:
[0042] (1) Weigh 2.21g (5.97mmol) of raw material 3-iodo-9-(3-pyridine)carbazole, 1.73g (17.97mmol) of sodium tert-butoxide powder, 0.12g (0.60mmol) of cuprous iodide, 0.13g (0.72mmol) of 1,10-phenanthroline was added to the flask reactor, and after nitrogen protection, anhydrous 80mL n-butanol solution was added; stirred at room temperature for 5min to fully dissolve the solid and disperse the system uniformly; then 0.77 g (6.03mmol) 4-thiophenol was added to the reaction system and continued to stir for 1min; the system was heated to 110°C and continued to reflux for 24h after the temperature stabilized; after the reaction, the crude product was added to 20mL dichloromethane and 100mL water, and Dichloromethane extraction (50mL×4); after removal of the solvent, a viscous liquid was obtained; the viscous liquid was dissolved in 30mL ethyl acetate and recrystallized at -...
Embodiment 2
[0047] Example 2 The preparation method for preparing the stimuli-responsive material is the same as that of Example 1, except that the raw material in step (1) is 2.21 g (5.97 mmol) of 3-iodo-9-(2-pyridine) carbazole; step (2) The raw material is 3-(4-fluorophenyl)mercapto-9-(2-pyridine)carbazole 2.12g (5.72mmol); the raw material of step (3) is 3-(4-fluorophenyl)sulfone-9-(2- Pyridine) carbazole 1.97g (4.89mmol), the structure is characterized as: 1 H NMR (400MHz, DMSO-d 6 ): δ9.16(d,1H),8.92(d,1H),8.83(dd,1H),8.56(d,1H),8.32(d,2H), 8.23(d,2H),8.18–8.10( m, 2H), 7.92 (d, 2H), 7.76 (dd, 1H), 7.59–7.54 (m, 2H), 7.47–7.38 (m, 6H), 7.30 (t, 2H).
[0048]
Embodiment 3
[0050] Example 3 The preparation method for preparing the stimuli-responsive material is the same as that of Example 1, except that the raw material in step (1) is 2.21 g (5.97 mmol) of 3-iodo-9-(4-pyridine) carbazole; step (2) The raw material is 2.12g (5.72mmol) of 3-(4-fluorophenyl)mercapto-9-(4-pyridine)carbazole; the raw material of step (3) is 3-(4-fluorophenyl)sulfone-9-(4- Pyridine) carbazole 1.97g (4.89mmol), the structure is characterized as: 1 H NMR (400MHz, DMSO-d 6 ): δ9.16(d,1H),8.92(d,1H),8.81(dd,1H),8.56(d,1H),8.32(d,2H), 8.23(d,2H),8.18–8.10( m, 2H), 7.92 (d, 2H), 7.76 (dd, 1H), 7.59–7.54 (m, 2H), 7.47–7.38 (m, 6H), 7.30 (t, 2H).
[0051]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com