Application of mesenchymal stem cell-derived small extracellular vesicles to preparation of drugs for treating autoimmune diseases
An autoimmune and mesenchymal stem cell technology, which is applied in small extracellular vesicles and the preparation of drugs for the treatment of autoimmune diseases, can solve the problems of mesenchymal stem cell dysfunction, unclear final differentiation fate, etc., to achieve The source is convenient to obtain, good rejection, low immunogenicity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1, Isolation and cultivation of gingival mesenchymal stem cells (GMSC)
[0050] 1. Collection of samples: collection of human gum samples, which were obtained from tissues discarded by patients undergoing routine dental surgery in the Department of Stomatology, Sun Yat-sen University Third Hospital, approved by the Medical Ethics Committee of the Sun Yat-sen University Third Hospital Ethics Committee (IRB) (IRB 2018-02-195-01).
[0051] 2. Isolation and culture of gingival mesenchymal stem cells (GMSC): the collected human gingival samples were digested with Disase II (2mg / mL) at 4°C overnight or 2h at 37°C; then with a concentration of 4mg / mL The IV-type collagenase was digested at 37°C for 0.5h (the tissue was shaken every few minutes to avoid debris); the cell suspension was collected by filtration with a 70μm sieve, centrifuged at 300g for 5min to obtain the cell pellet, and the cell pellet was washed with α - MEM complete medium (containing 10% FBS, 100...
Embodiment 2
[0054] Example 2, Isolation and identification of GMSC-sEV
[0055] Take the supernatant of the resuspension prepared in Step 2 of Example 1 and centrifuge at 300g for 5min to remove cells; centrifuge at 3,000g for 15min to remove dead cells; centrifuge at 10,000g for 30min to remove cell debris; centrifuge at 110,000g for 70min to remove the supernatant, and The pellet is a mixture containing protein and EVs; then resuspend the pellet with a large volume of PBS and continue to centrifuge at 110,000g for 70min, the final pellet is small EVs (GMSC-sEV); the final sEV is resuspended in PBS and used immediately or Store at -20°C.
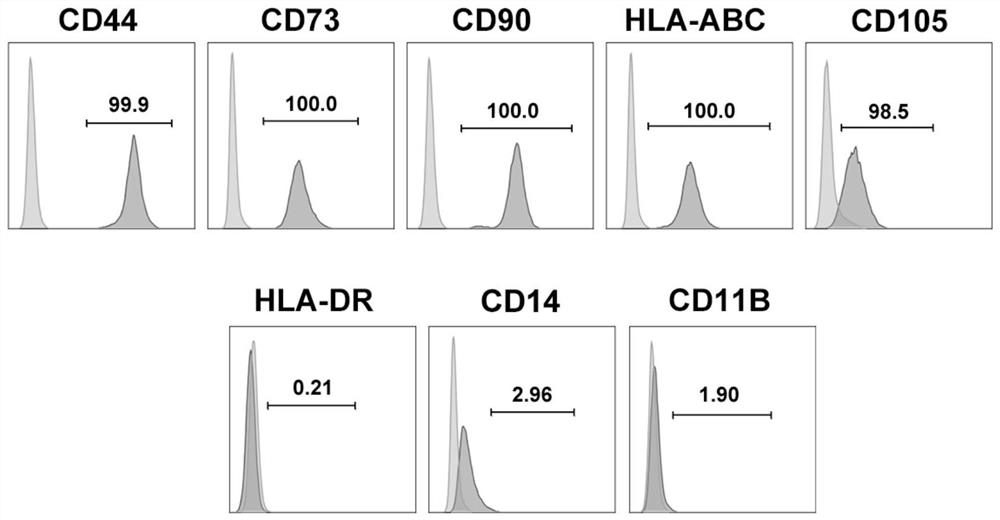
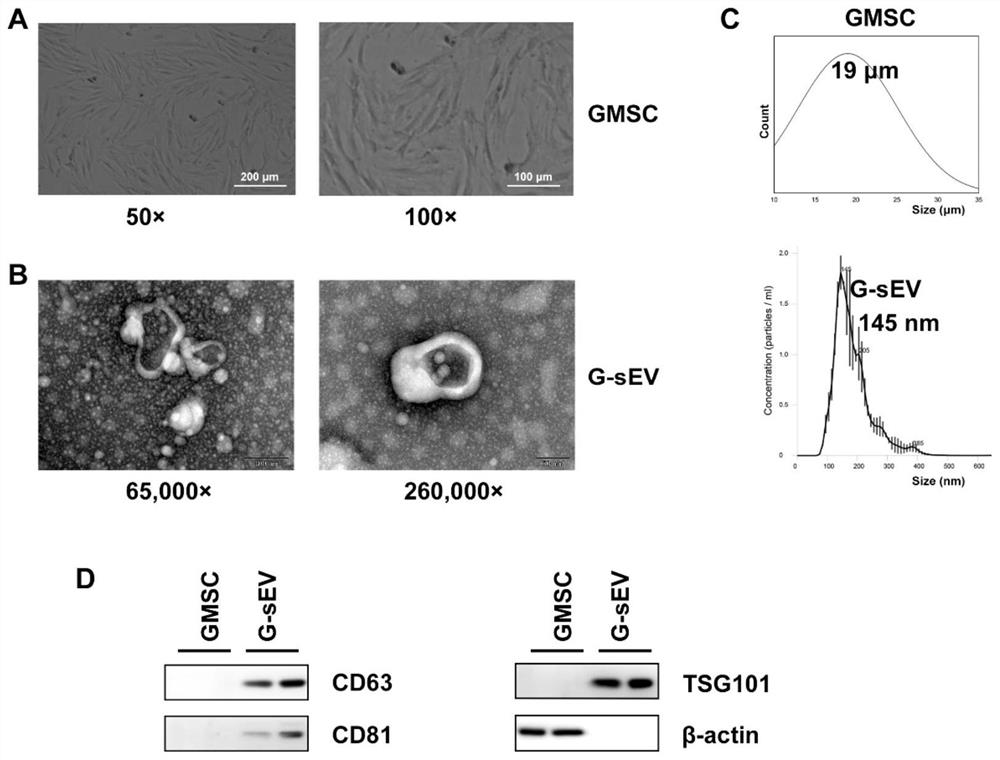
[0056] Obtained GMSCs were observed under a microscope, such as figure 2 -A, the diameter of GMSC is distributed in 10-30 μm, and the obtained GMSC-sEV is detected by transmission electron microscope (TEM), such as figure 2 -B, the diameter size distribution of GMSC-sEV is at 120nm, further adopts nanoparticle tracking analysis (NTA) technology to ...
Embodiment 3
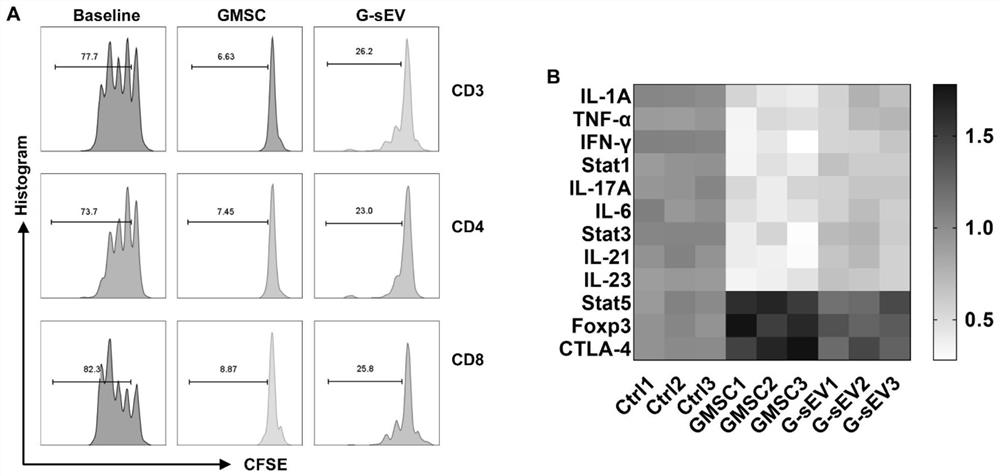
[0057] Example 3, Detection of GMSC-sEV inhibiting T cell proliferation in vitro
[0058] Take the whole blood from healthy volunteers and dilute it with an equal volume of PBS and mix it evenly, then slowly add the diluted whole blood ( Be careful not to damage the interface of the separation liquid layer), room temperature (18-28°C), 400g, centrifuge for 20-30min (the centrifuge is set to acceleration 3, deceleration 0). After the centrifugation is over, carefully aspirate the cells in the buffy coat. All the collected cells were then centrifuged with 5 times the volume of PBS at room temperature at 300 g for 10 min, and washed 1-2 times with PBS to remove platelets as much as possible. The final obtained hPBMCs (human peripheral blood mononuclear cells) were used immediately or stored at -80°C with frozen storage solution for later use.
[0059] hPBMCs obtained freshly or revived one night in advance were placed in 1 μM CFSE (carboxyfluoresceindiacetate succinimidyl ester...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com