A kind of berberine derivative and its preparation method and its application as p300 HAT small molecule inhibitor
A technology of small molecule inhibitors and derivatives, applied in anti-inflammatory agents, drug combinations, antiviral agents, etc., can solve the problems of high cytotoxicity, weak affinity, and low activity, and achieve simple synthetic routes and improved enzyme level activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
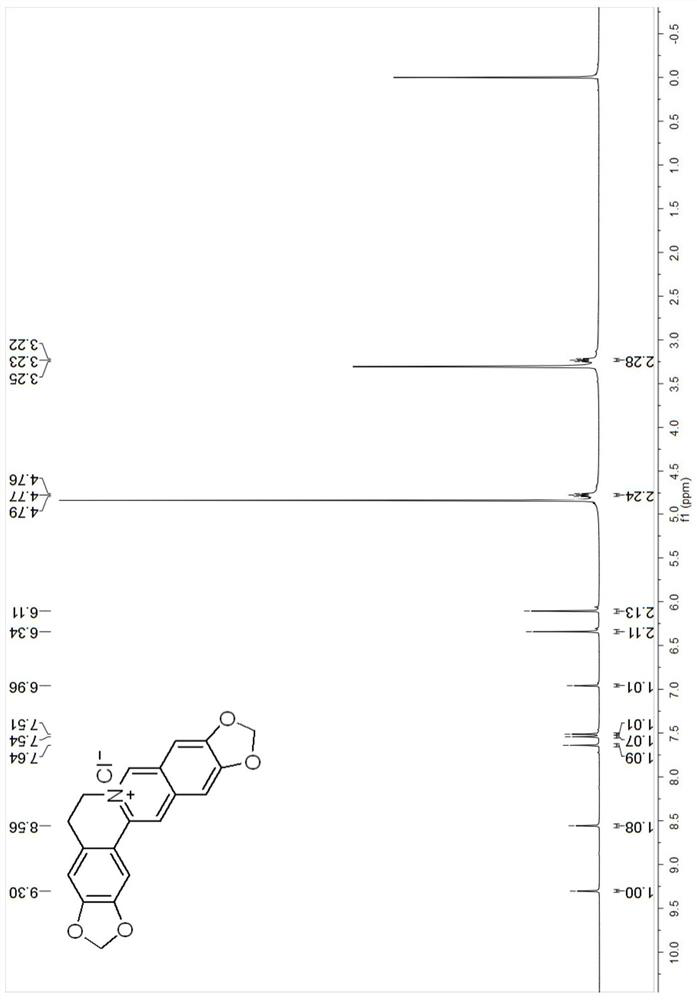
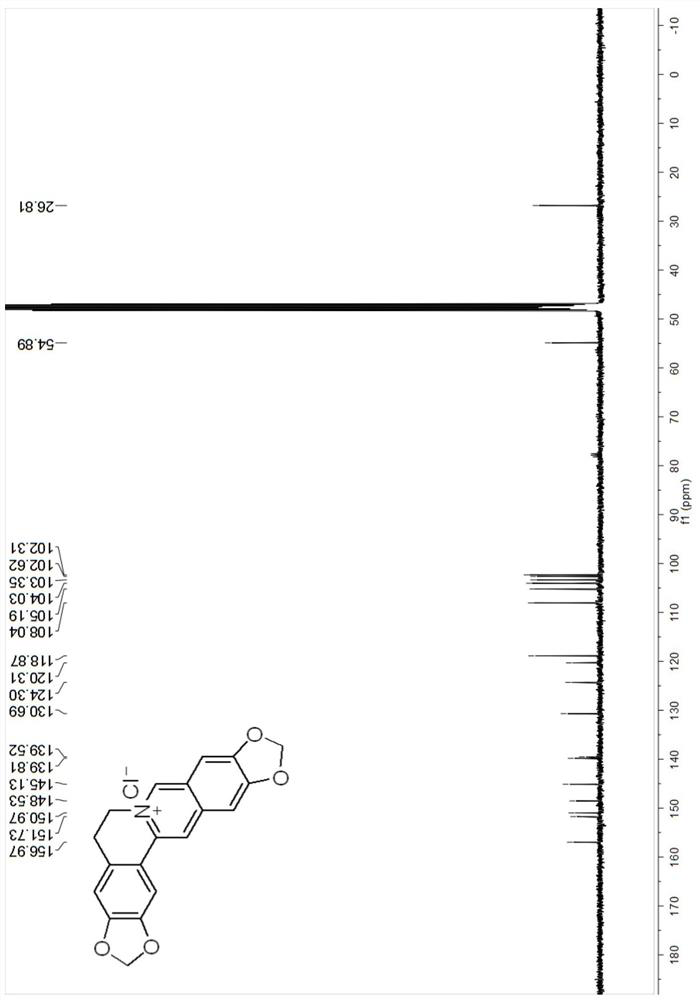
[0049] The berberine derivative compound Cpd.1 provided in Example 1, its synthesis schematic and steps are as follows:
[0050]
[0051]Preparation of nitrostyrene derivative B-1: Mix substituted benzaldehyde compound A-1 (0.0667 mol), ammonium acetate (0.130 mol), nitromethane (0.165 mol) and glacial acetic acid (11ml), and heat at 100°C Next, the reaction was stirred for 3 hours. Cool to room temperature, add water (20ml), filter, wash the filter residue with water until neutral, and dry to obtain compound B-1;
[0052] Preparation of substituted phenethylamine hydrochloride C-1: In freshly prepared zinc amalgam (50g), add compound B-1 (10g) and 95% ethanol (400ml), add concentrated hydrochloric acid (80ml) under stirring , stirred at room temperature for 1 hour, filtered, the filtrate was concentrated under reduced pressure to 140-150ml, alkalized with concentrated ammonia water to pH 9-10, extracted with chloroform, washed with water, dried over anhydrous magnesium su...
Embodiment 2
[0057] The synthetic diagram and steps of the berberine derivative compound Cpd.2 provided in Example 2 differ from Example 1 only in that the synthetic raw material substituted benzaldehyde compound D-1 is replaced with substituted benzaldehyde compound D-2 .
[0058] The structural formulas of substituted benzaldehyde compound D-2 and compound Cpd.2 are as follows:
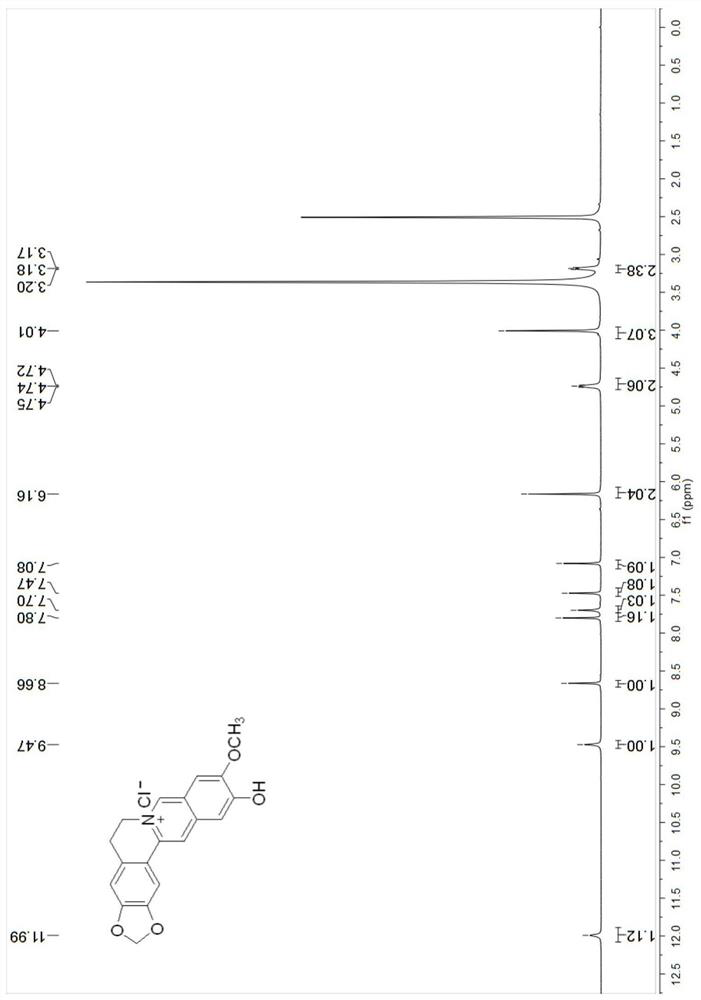
[0059]
[0060] The NMR spectrum of compound Cpd.2 is as follows Figure 3-4 As shown, NMR characterization data: 1 H NMR (400MHz, DMSO-d 6 )δ11.99(s,1H),9.47(s,1H),8.66(s,1H),7.80(s,1H),7.70(s,1H),7.47(s,1H),7.08(s,1H ), 6.16(s, 2H), 4.74(t, J=6.1Hz, 2H), 4.01(s, 3H), 3.18(t, 2H). 13 C NMR (101MHz, DMSO-d 6 )δ157.7, 152.8, 148.1, 145.7, 138.2, 137.2, 131.0, 122.0, 121.2, 117.9, 108.9, 108.8, 107.8, 106.0, 102.5, 56.8, 54.8, 27.1. HRMS (ESI) m / z: Calcd for C 19 h 16 NO 4 + [M] + 322.1074, fo μnd 322.1074.
Embodiment 3
[0062] The synthetic diagram and steps of the berberine derivative compound Cpd.3 provided in Example 3 differ from Example 1 only in that the synthetic raw material substituted benzaldehyde compound A-1 is replaced by substituted benzaldehyde compound A-3 , the synthetic raw material substituted benzaldehyde compound D-1 was replaced by substituted benzaldehyde compound D-3.
[0063] The structural formulas of substituted benzaldehyde compound A-3, substituted benzaldehyde compound D-3 and compound Cpd.3 are as follows:
[0064]
[0065] The NMR spectrum of compound Cpd.3 is as follows Figure 5-6 As shown, NMR characterization data: 1 H NMR (400MHz, Methanol-d 4 )δ 9.33(s,1H),8.68(s,1H),7.66(s,1H),7.55(d,J=7.2Hz,2H),7.07(s,1H),6.36(s,2H),4.81 (t,J=6.4Hz,2H),4.01(s,3H),3.96(s,3H),3.28(t,J=6.5Hz,2H). 13 C NMR (101MHz, Methanol-d 4 )δ156.9, 152.6, 151.6, 149.4, 145.1, 139.8, 139.6, 128.8, 124.2, 118.8, 118.6, 110.8, 108.5, 104.0, 103.3, 102.5, 55.6, 55.3, 55.0, 26, 4. H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com