Berberine derivatives, preparation method thereof and application of berberine derivatives as p300 HAT small molecule inhibitor
A derivative, the technology of berberine, applied in the field of medicinal chemistry, can solve the problems of weak affinity, high cytotoxicity, poor selectivity, etc., and achieve the effect of improved enzyme level activity and simple synthetic route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
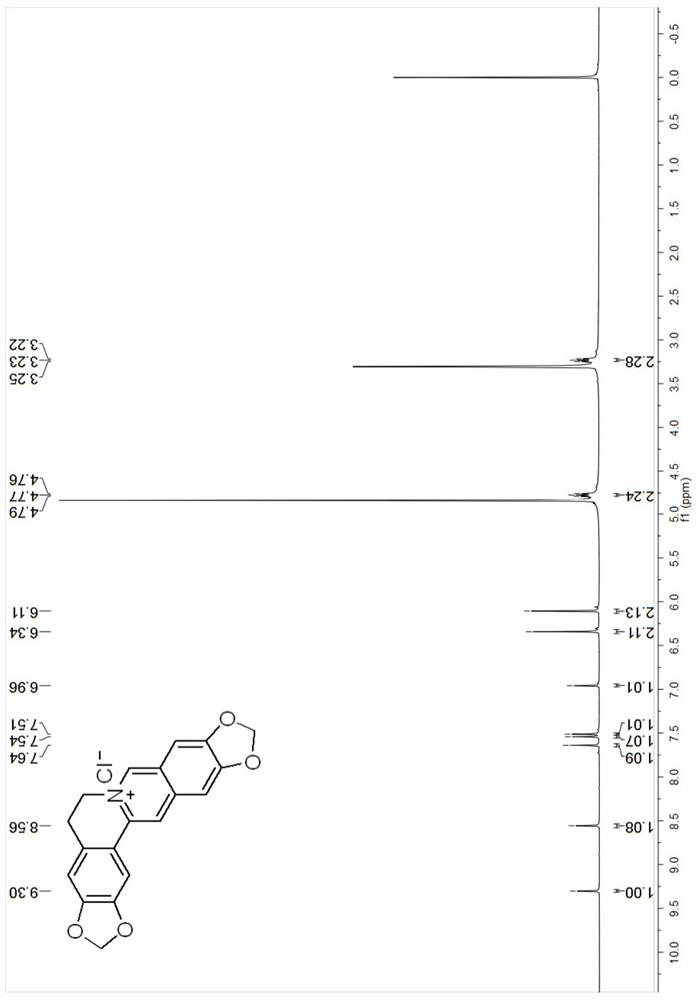
[0049]The compound Cpd.1 of the berberine derivative provided in Example 1, its synthesis schematic and steps are as follows:
[0050]
[0051]Preparation of nitrostyrene derivative B-1: Mix the substituted benzaldehyde compound A-1 (0.0667mol), ammonium acetate (0.130 mol), nitromethane (0.165mol) and glacial acetic acid (11ml) at 100℃ Next, the reaction was stirred for 3 hours. Cool to room temperature, add water (20ml), filter, wash the filter residue with water to neutrality, and dry to obtain compound B-1;
[0052]Preparation of substituted phenethylamine hydrochloride C-1: To freshly prepared zinc amalgam (50g), add compound B-1 (10g) and 95% ethanol (400ml), add concentrated hydrochloric acid (80ml) under stirring , Stirred at room temperature for 1 hour, filtered, the filtrate was concentrated under reduced pressure to 140~150ml, basified with concentrated ammonia water to pH 9~10, extracted with chloroform, washed with water, dried with anhydrous magnesium sulfate, evaporated under ...
Embodiment 2
[0057]The compound Cpd.2 of the berberine derivative provided in the present example 2 is different from the example 1 in the synthesis schematic diagram and the steps: the synthetic raw material substituted benzaldehyde compound D-1 is replaced with substituted benzaldehyde compound D-2 .
[0058]The structural formulas of substituted benzaldehyde compound D-2 and compound Cpd.2 are as follows:
[0059]
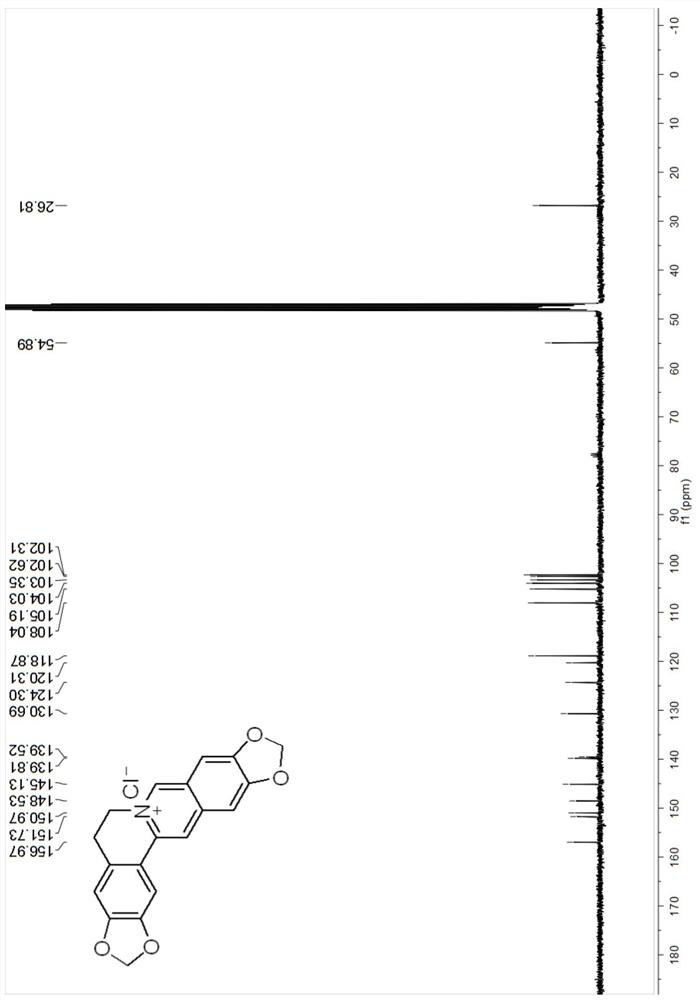
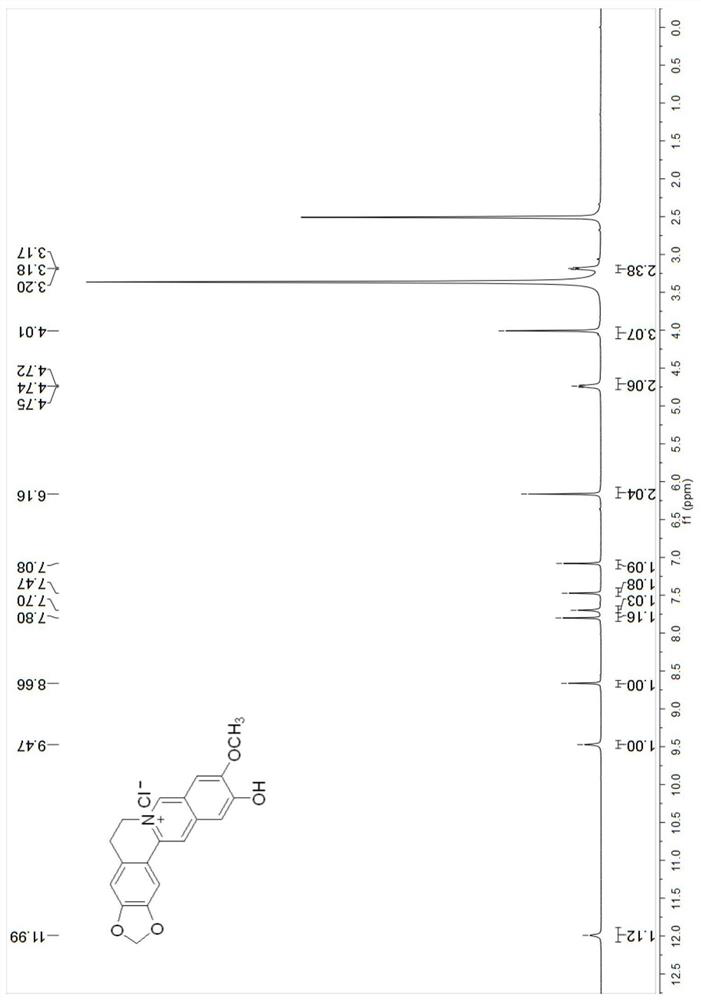
[0060]The NMR spectrum of compound Cpd.2 is as followsFigure 3-4 As shown, the NMR characterization data:1H NMR(400MHz, DMSO-d6)δ11.99(s, 1H), 9.47(s, 1H), 8.66(s, 1H), 7.80(s, 1H), 7.70(s, 1H), 7.47(s, 1H), 7.08(s, 1H) ), 6.16(s, 2H), 4.74(t, J=6.1Hz, 2H), 4.01(s, 3H), 3.18(t, 2H).13C NMR(101MHz, DMSO-d6)δ157.7, 152.8, 148.1, 145.7, 138.2, 137.2, 131.0, 122.0, 121.2, 117.9, 108.9, 108.8, 107.8, 106.0, 102.5, 56.8, 54.8, 27.1.HRMS(ESI)m / z:Calcd for C19H16NO4+[M]+322.1074, foμnd 322.1074.
Embodiment 3
[0062]The berberine derivative compound Cpd.3 provided in this example 3, its synthesis schematic and steps are different from Example 1 only in that: the synthetic raw material substituted benzaldehyde compound A-1 is replaced with substituted benzaldehyde compound A-3 , The synthetic raw material substituted benzaldehyde compound D-1 was replaced with substituted benzaldehyde compound D-3.
[0063]The structural formulas of substituted benzaldehyde compound A-3, substituted benzaldehyde compound D-3 and compound Cpd.3 are as follows:
[0064]
[0065]The NMR spectrum of compound Cpd.3 is as followsFigure 5-6 As shown, the NMR characterization data:1H NMR(400MHz, Methanol-d4)δ 9.33(s,1H),8.68(s,1H),7.66(s,1H),7.55(d,J=7.2Hz,2H),7.07(s,1H),6.36(s,2H),4.81 (t,J =6.4Hz,2H),4.01(s,3H),3.96(s,3H), 3.28(t,J=6.5Hz,2H).13C NMR(101MHz, Methanol-d4)δ156.9,152.6,151.6,149.4,145.1,139.8,139.6,128.8,124.2,118.8,118.6,110.8, 108.5,104.0,103.3,102.5,55.6,55.3,55.0,26,4.HRMS(ESI)m / z :Calcd for C20H18NO4+[M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com