Application of a naringenin (4-o-methyl) glucoside compound in the preparation of anti-inflammatory or lipid-lowering drugs
A technology of glucoside and anti-inflammatory drugs, which is applied in the field of application of naringenin glucoside compounds in anti-inflammatory or lipid-lowering drugs, and can solve the problem of lipid-lowering activity of methyl glycosidation derivatives which are rarely reported, naringenin The anti-inflammatory activity of derivatives is rarely reported and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1 Methyl glycosylation modification of naringenin by Isaria fumosorosea ACCC 37814
[0019] 1.1 Instruments and Materials
[0020] The strain used in the experiment: Isaria fumosorosea ACCC 37814 was obtained from China Agricultural Microorganism Collection Center (ACCC). Compounds: purchased from Shanghai Yuanye Biotechnology Co., Ltd. (Shanghai, China). The reagents used in the experiments were all domestic analytically pure products. Medium: The fungal medium is potato dextrose agar (PDA, Difco, Sparks, MD, USA) medium and potato dextrose broth (PDB, Difco, Sparks, MD, USA) medium, if solid medium, add 2 % agar powder.
[0021] High performance liquid chromatography (Agilent, Agilent 1260 Infinity II HPLC and Agilent 1290 Infinity II HPLC), C 18Chromatographic columns (Agilent, Poroshell 120SB-C18, 2.7 μm, 4.6 mm×150 mm and Zorbax SB-C18, 1.8 μm, 2.1×50 mm). Mass spectrometry (Agilent, Agilent QTOF 6530). Rotary evaporator (BUCHI company, DL-400); circu...
Embodiment 2
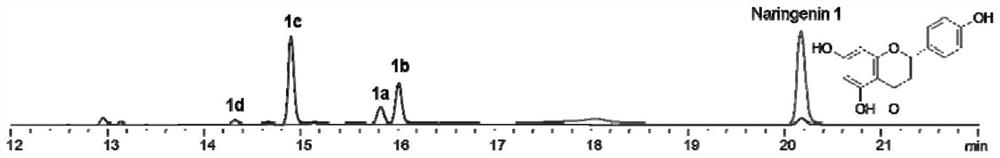
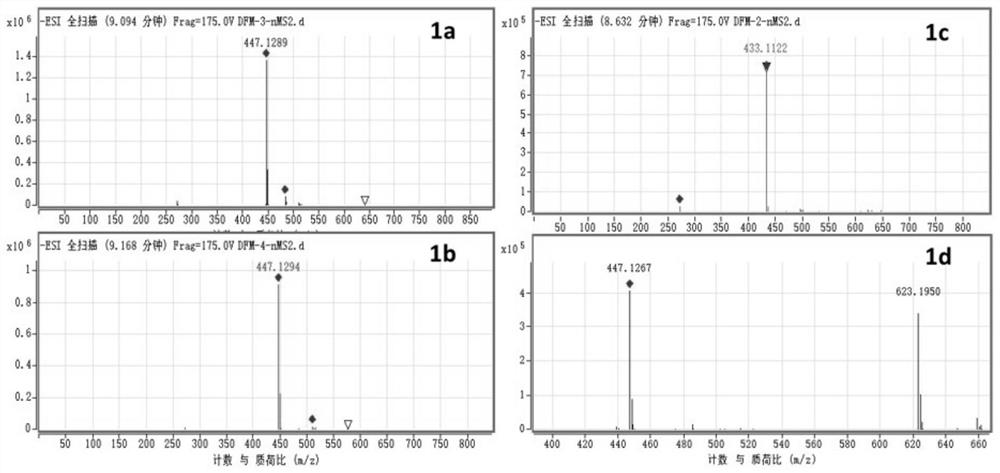
[0030] Example 2 Extraction, isolation and structural identification of compounds 1a-1d
[0031] 2.1 Instruments and materials
[0032] Chromatographic pure water, chromatographic acetonitrile, analytically pure ethyl acetate, chromatographic or ordinary pure methanol, dichloromethane, etc. used in the laboratory were purchased from Fisher (USA) Company; column chromatography silica gel (100-200 mesh) was purchased from Qingdao Ocean chemical plant company. BUCHIDL-400 type rotary evaporator, produced by Swiss BUCHI company. The circulating cooler is produced by Zhengzhou Great Wall Technology Industry and Trade Co., Ltd. The semi-automatic preparative liquid chromatograph was Agilent 1260Infinity II, and the C18 chromatographic column was Eclipse XDB-C18, 5 μm, 9.4×250 mm. Sample purity testing was performed using an Agilent 1260 Infinity II analytical HPLC instrument equipped with a Poroshell 120SB-C18 reversed-phase column (2.7 μm, 4.6 mm×150 mm). The detection conditio...
Embodiment 3
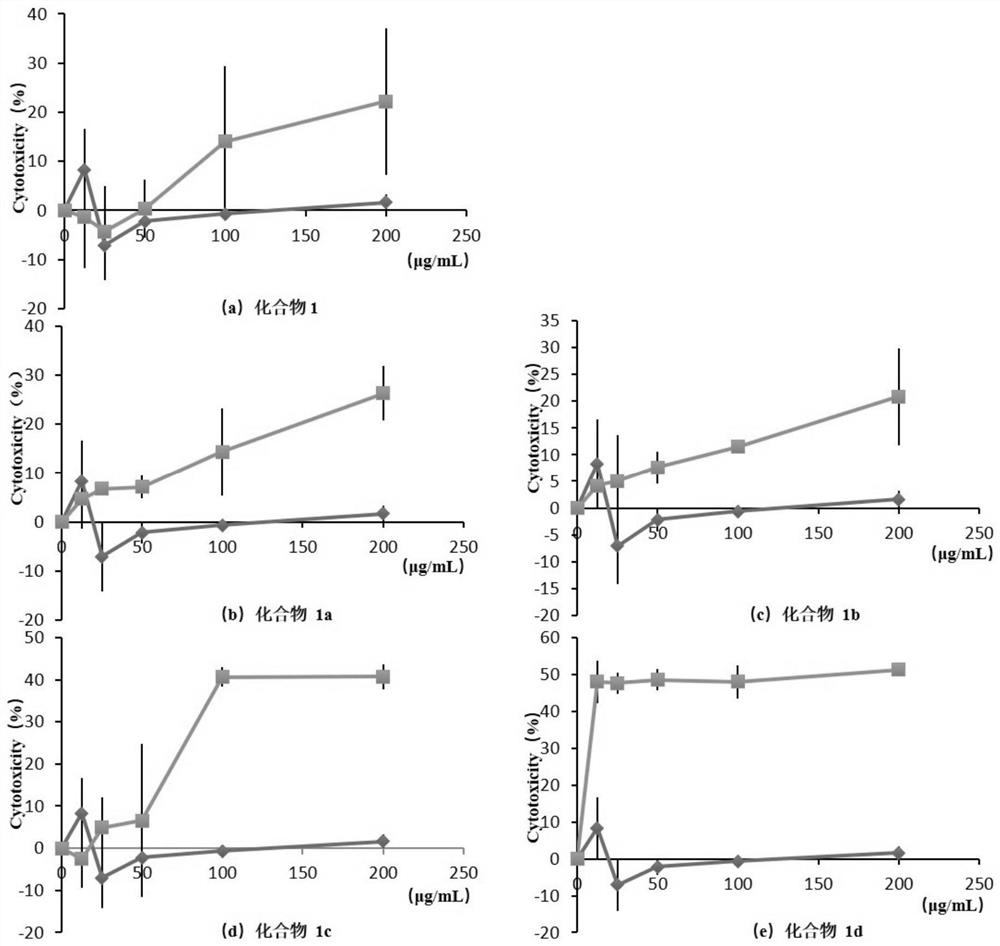
[0050] Embodiment 3 Solubility test of naringenin and its glycoside compounds
[0051] 3.1 Experimental purpose
[0052] To determine the effect of glycomethylation on the solubility of naringenin in different solvents.
[0053] 3.2 Experimental method
[0054] Weigh 0.5 mg of naringenin and compounds 1b and 1c respectively, put them in 500uL of different solvents at 25°C ± 2°C, and shake vigorously for 30s every 5min; observe the dissolution within 30min, if no solute particles or liquid are visible. When dripping, it is considered to be completely dissolved. The selected solvent includes distilled water, methanol, ethanol and the like.
[0055] 3.3 Experimental results and analysis
[0056] The solubility of naringenin and its glycosylated derivatives (compound 1c) and sugar methylated derivatives (compound 1b) in different solvents is shown in Table 3.
[0057] Table 3 Solubility of Naringenin, Compounds 1b and 1c in Different Solvents
[0058]
[0059]
[0060]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com