Amorphous composite material as well as preparation method and application thereof
A technology of amorphous composite materials and amorphous materials, which is applied in the field of high-selectivity 2e-oxygen reduction electrocatalyst materials and its preparation, can solve the problems of inapplicability to large-scale production, and achieve simple methods, easy commercial production, Environmentally friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Example 1 Sample 1 Preparation
[0067] A-Cu(OH) 2 / GO preparation process:
[0068] 1). Dissolution of copper ions;
[0069] CuCl 2 2H 2 O and 16mg of graphene oxide were dissolved in a mixed solution of 40ml of water and ethylene glycol (the volume ratio of ethylene glycol and water was 4:1), stirred to form 0.006M CuCl 2 Mix the solution uniformly.
[0070] 2). The solvent controls the precipitation process;
[0071] Slowly add 120 ul of ammonia water with a concentration of 28% mass fraction to the homogeneous mixed solution, adjust the pH to pH=9, stir for 10 minutes, centrifuge wash three times with deionized water, and freeze-dry to obtain a solid powder sample, which is designated as sample 1.
Embodiment 2
[0072] Embodiment 2 Sample 2~4 preparation
[0073] Concrete operation is the same as embodiment 1, and its difference is, adopt the Cu(NO of 0.001M 3 ) 2 The solution was mixed uniformly, and the prepared sample was designated as sample 2.
[0074] Concrete operation is the same as embodiment 1, and its difference is, adopt the CuCl of 0.001M 2 The solution was uniformly mixed, and the prepared sample was designated as sample 3.
[0075] Concrete operation is the same as embodiment 1, and its difference is, adopt the CuCl of 0.007M 2 The solution was uniformly mixed, and the prepared sample was designated as sample 4.
Embodiment 3
[0076] Example 3 Structural Characterization
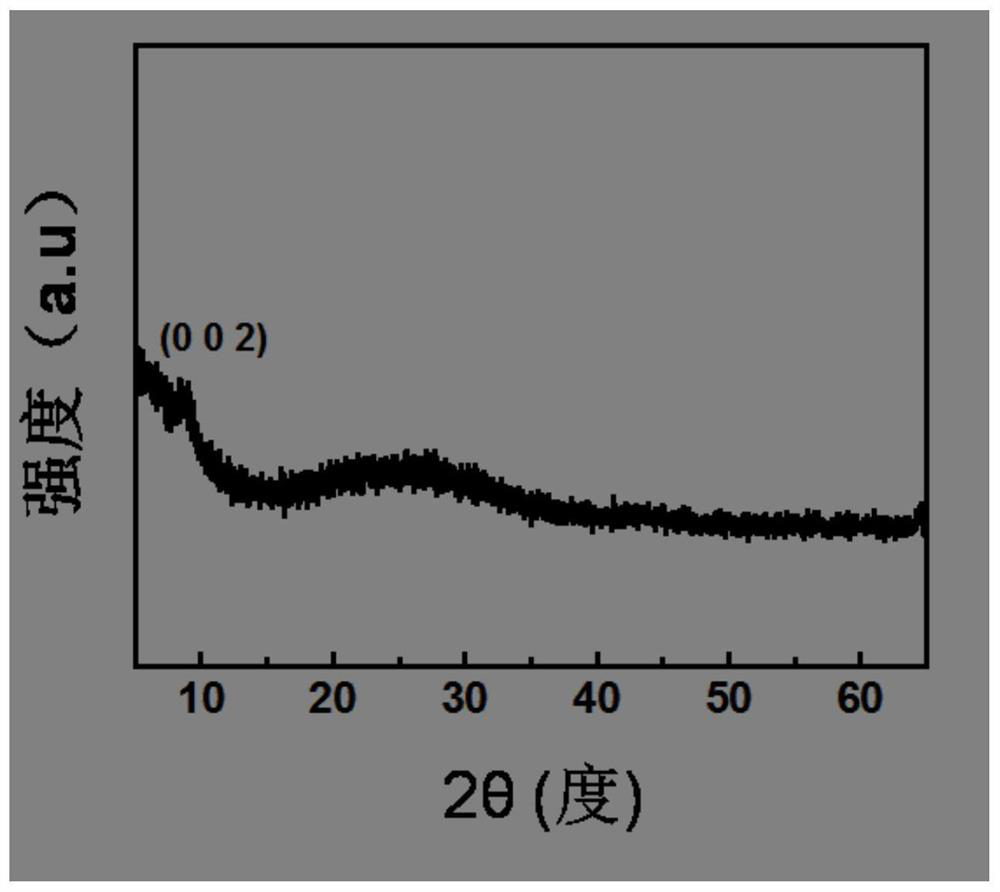
[0077] Carry out XRD test on samples 1-4, the typical XRD spectrum is as follows figure 1 Shown, corresponding to the sample 1 in the embodiment 1. figure 1 It is shown that the prepared sample has only one obvious graphene oxide (002) plane, and there are no other obvious diffraction peaks, which indicates that the prepared sample is an amorphous phase. XRD spectra of samples 2-4 and figure 1 Similar, the only difference lies in the intensity of the diffraction peaks.
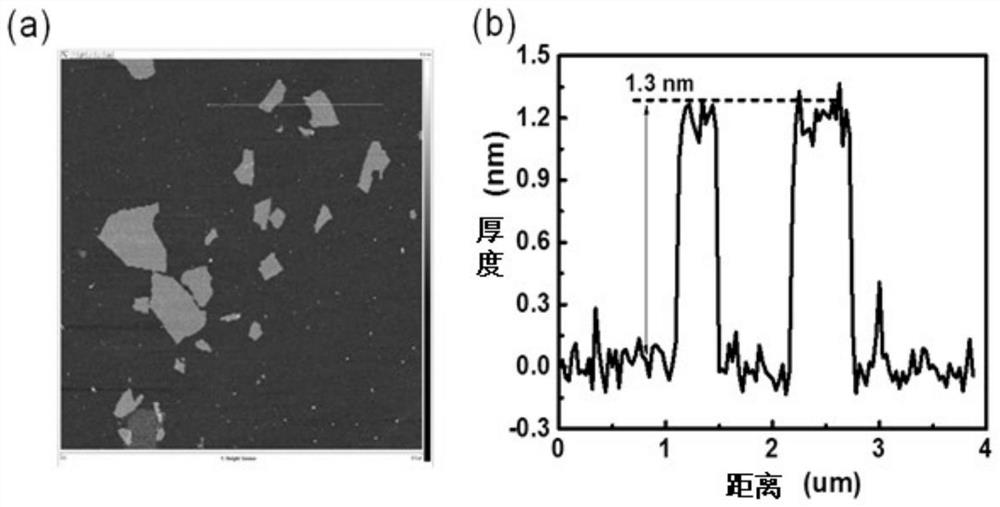
[0078] Perform AFM and corresponding height distribution tests on samples 1 to 4, typical AFM such as figure 2 As shown in (a), a typical height distribution map is as follows figure 2 Shown in (b), corresponds to sample 1 in Example 1. figure 2 (a) and figure 2 (b) shows that the thickness of graphene oxide is about 1.3 nm. AFM and height distribution maps of samples 2-4 and figure 2 (a) and figure 2 (b) similar.
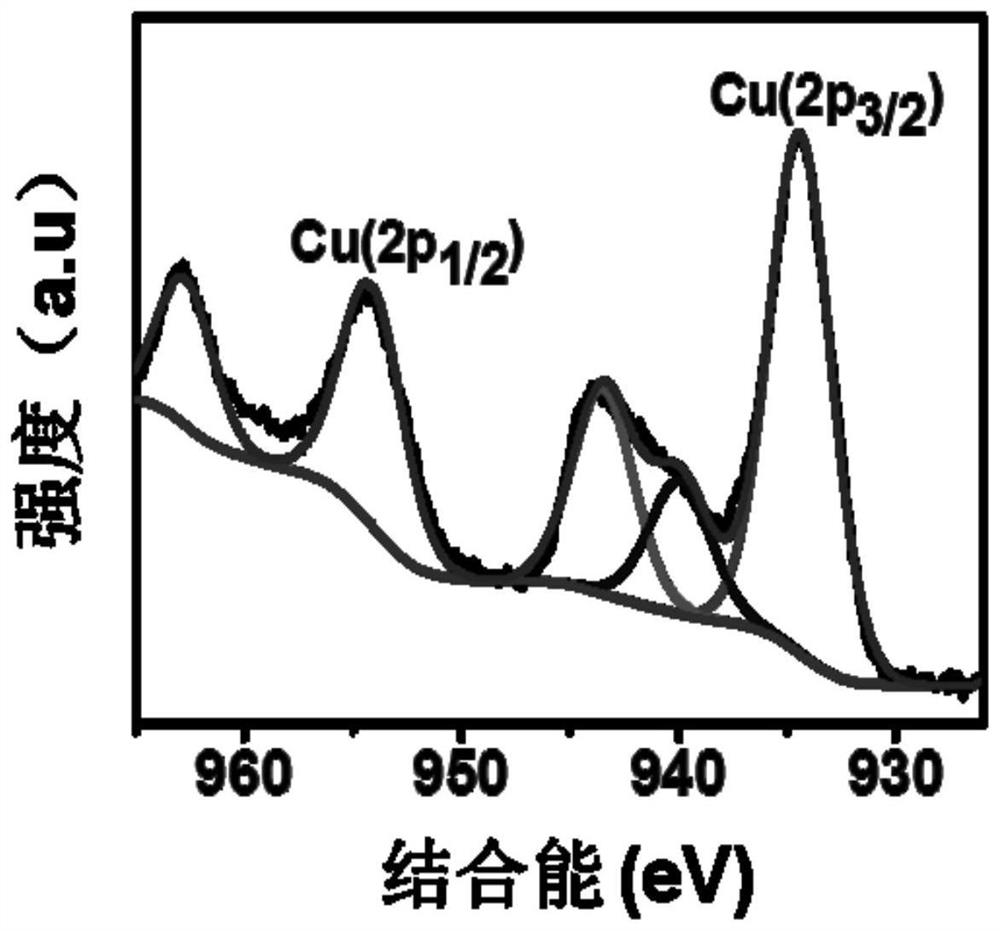
[0079] Carry out XPS test on sam...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com