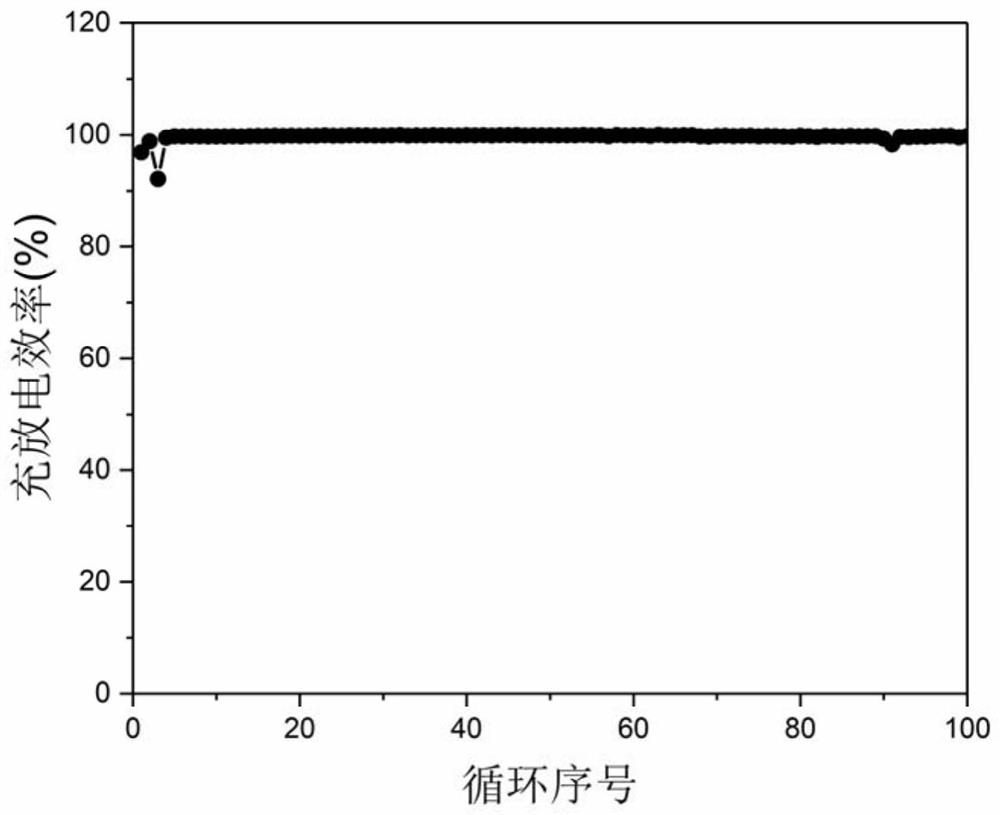
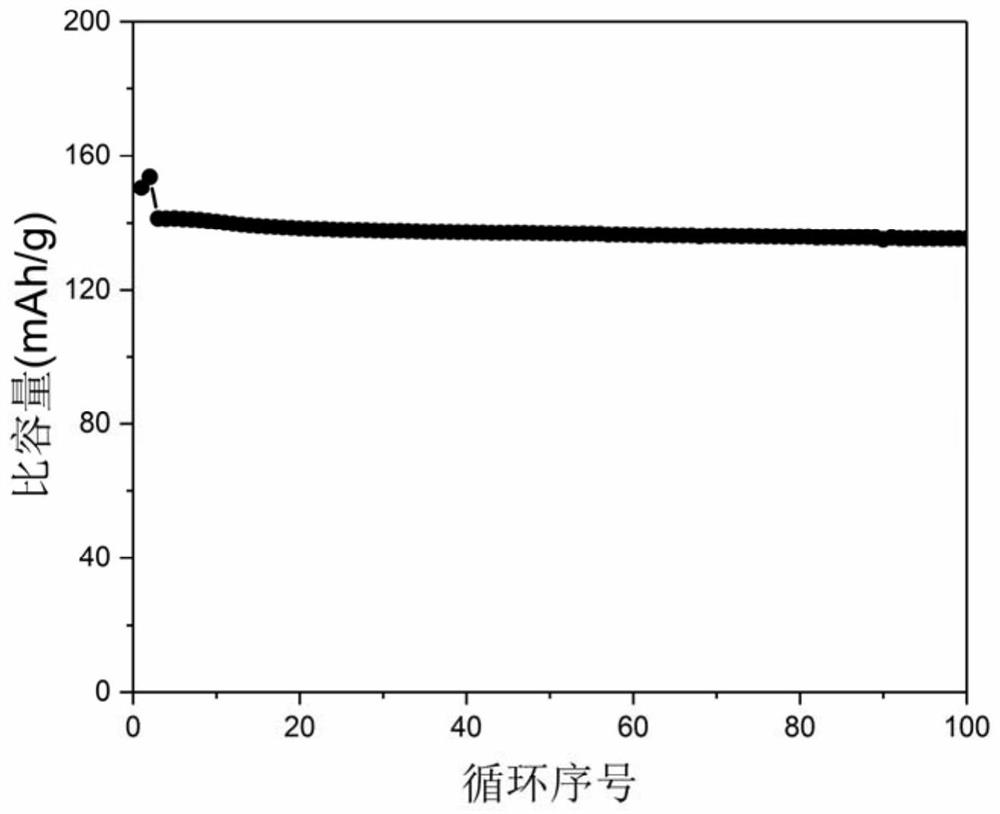
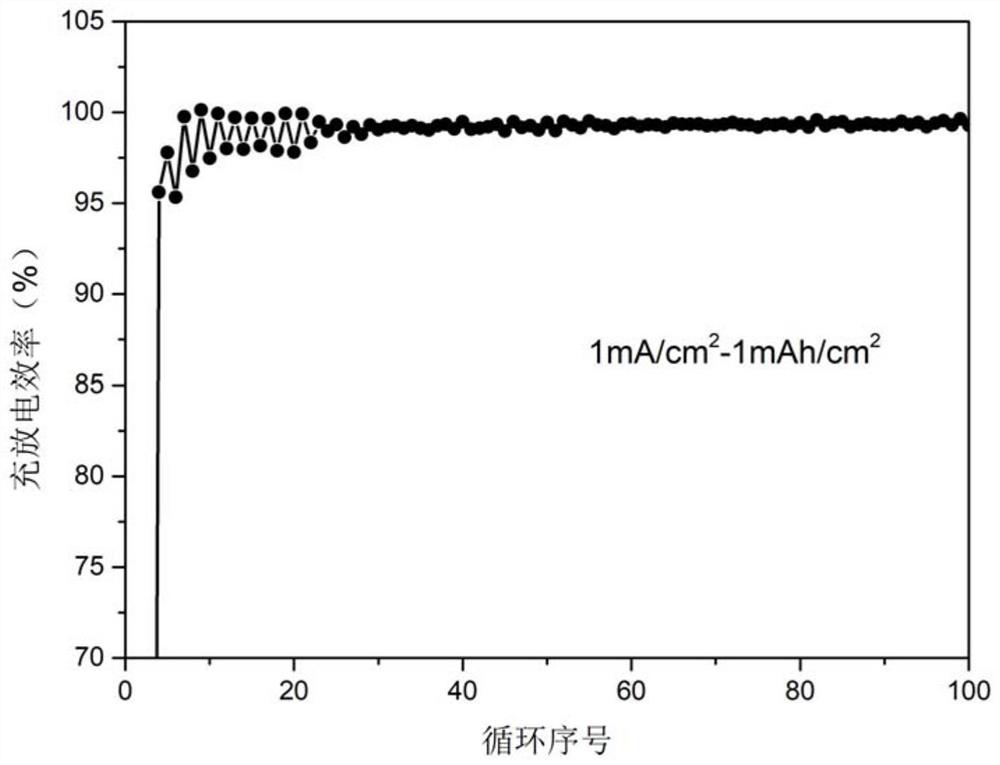
Fluorine-containing ether electrolyte cosolvent for lithium metal/lithium ion/lithium sulfur battery, electrolyte and lithium secondary battery
A lithium-sulfur battery, fluorine-containing ether technology, applied in secondary batteries, secondary battery repair/maintenance, lithium batteries, etc., can solve problems such as poor stability and low oxidation decomposition potential, reduce flammability, and improve oxidation resistance. Potential, the effect of suppressing the growth of lithium dendrites
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] Correspondingly, the embodiment of the present invention also provides a preparation method of fluorine-containing ether compounds, which includes the following steps:
[0041] S1. Provide p-toluenesulfonyl chloride, fluoroalcohol and alkali metal hydroxide; mix p-toluenesulfonyl chloride, fluoroalcohol and alkali metal hydroxide, and p-toluenesulfonyl chloride and fluoroalcohol generate p-toluenesulfonate.
[0042] S2. Mixing the p-toluenesulfonate with fluoroalcohol and alkali metal hydroxide, the fluoroalcohol and the alkali metal hydroxide react to form a fluoroalcohol organic base, the fluoroalcohol organic base and the p-toluenesulfonate A bimolecular nucleophilic substitution reaction occurs to generate the fluorine-containing ether compound (named DFE);
[0043] The molecular structural formulas of fluorine-containing ether compounds are shown in formulas (I)-(VII):
[0044]
[0045]
[0046] In the preparation method of the fluorine-containing ether comp...
Embodiment 1
[0065] A preparation method for fluorine-containing ether compounds, comprising the steps of:
[0066] (1) Dissolve 0.214mol of 2,2-difluoroethanol (its molecular structure is shown in formula (i)) in 65mL of tetrahydrofuran, and add 65mL of 6M NaOH solution dropwise under ice-bath conditions to generate 2,2 -Sodium difluoroethanolate solution; then dissolve 0.22mol of p-toluenesulfonyl chloride in 50mL tetrahydrofuran, add the above-mentioned sodium 2,2-difluoroethoxide solution under ice bath and stirring conditions, and stir and react under ice bath conditions for 1 hour , rise to room temperature, continue to stir to make it fully react for 1.5 hours, then add 170mL of NaOH solution (1M) to convert excess p-toluenesulfonyl chloride into a water-soluble salt for easy removal; add 200mL of ether for extraction, and wash with ultrapure water Three times, under reduced pressure to spin off the ether to obtain 2,2-difluoroethyl p-toluenesulfonate;
[0067] (2) Dissolve 2,2-dif...
Embodiment 2
[0070] A preparation method for fluorine-containing ether compounds, comprising the steps of:
[0071] (1) Dissolve 0.214mol of 2-fluoroethanol (its molecular structure is shown in formula (ii)) in 65mL of tetrahydrofuran, and add 65mL of 6M NaOH solution dropwise under ice-bath conditions to generate sodium 2-fluoroethoxide solution ; Then 0.25mol of p-toluenesulfonyl chloride was dissolved in 50mL of tetrahydrofuran, and added to the above-mentioned sodium 2-fluoroethoxide solution under ice bath and stirring conditions, stirred and reacted for 1.5 hours under ice bath conditions, rose to room temperature, and continued to stir to make it Fully react for 2 hours, then add 170mL of NaOH solution (1M) to convert the excess p-toluenesulfonyl chloride into a water-soluble salt for easy removal; then add 200mL of ether for extraction, wash with ultrapure water three times, and spin off the ether under reduced pressure to obtain 2-fluoroethyl p-toluenesulfonate;
[0072] (2) Diss...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com