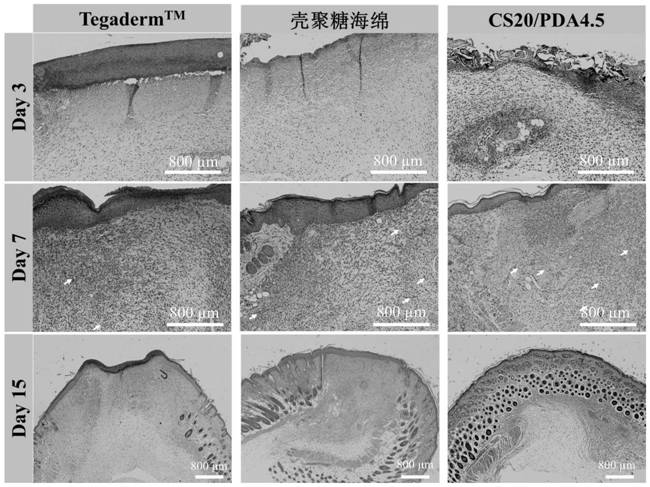
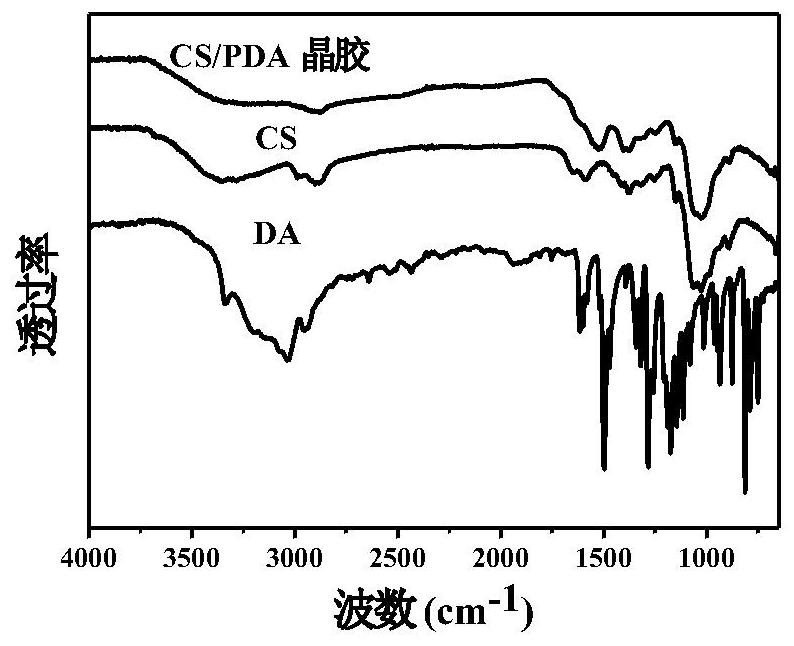
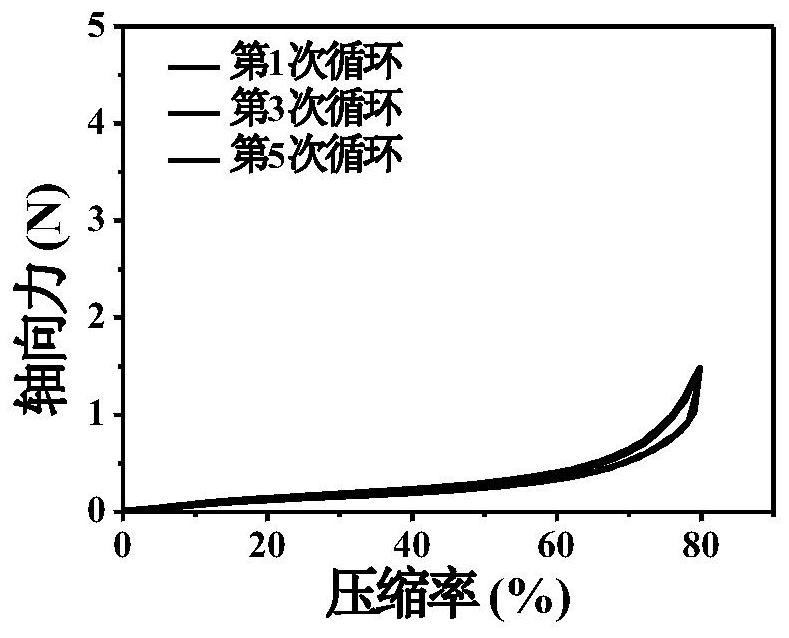
Injectable and degradable dry hemostatic cryogel with good shape memory and blood coagulation capability as well as preparation method and application of injectable and degradable dry hemostatic cryogel
A technology of blood coagulation and crystal gel, which is applied in the field of biomedical materials to achieve the effects of rapid blood concentration, accelerated coagulation, and the formation of blood vessels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] The preparation method of the present invention comprises the following steps:
[0045] 1) Resuspend chitosan (CS) in deionized water, then add glacial acetic acid dropwise with stirring, and then heat and stir at 50-60°C for 30-60 minutes to obtain CS solution; in CS solution, chitosan, deionized water The ratio between glacial acetic acid and glacial acetic acid is 1.0g:(39~160)mL:(1000~4000)μL; add dopamine hydrochloride (DA) into deionized water to make DA solution;
[0046] 2) After the CS solution, DA solution and oxidant solution are pre-cooled in an ice bath, mix the CS solution and DA solution, and at the same time add the oxidant solution and mix evenly to obtain a mixed solution, wherein the final mass concentration of CS is 0.5~ 2.0%, the final concentration of DA is 0.5-9 mg / mL; the oxidizing agent is sodium periodate; the molar ratio of sodium periodate to dopamine is 0.5-1:1;
[0047] 3) Put the mixed solution at -7~-20°C for 12~36h to obtain the frozen ...
Embodiment 1
[0052] Preparation of CS20 / PDA0.5 crystal gel: resuspend chitosan in deionized water, then add glacial acetic acid dropwise with stirring, and then heat and stir at 55°C for 40min to obtain 2.5wt% CS solution. In CS solution, chitosan The ratio between sugar, deionized water and glacial acetic acid is 1.0g: 39mL: 1000μL; add dopamine hydrochloride to deionized water to make a 5mg / mL DA solution; add sodium periodate to deionized water to make a 5.64 mg / mL SP solution; the molar ratio of sodium periodate to dopamine is 1:1. Subsequently, the CS solution, the DA solution and the SP solution were fully pre-cooled in an ice-water mixing bath and then fully mixed to obtain a final concentration of CS of 2.0 wt % and a final concentration of DA of 0.5 mg / mL. Then the mixture was placed in a low-temperature reactor at -20°C for 36 h. After the reaction, the crystal gel was melted in deionized water and freeze-dried at -80°C to obtain the injectable and degradable dry crystal gel hem...
Embodiment 2
[0054] The final concentration of DA in the step was controlled at 1.5 mg / mL, and other conditions were the same as in Example 1 to obtain CS20 / PDA1.5 crystal gel.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com