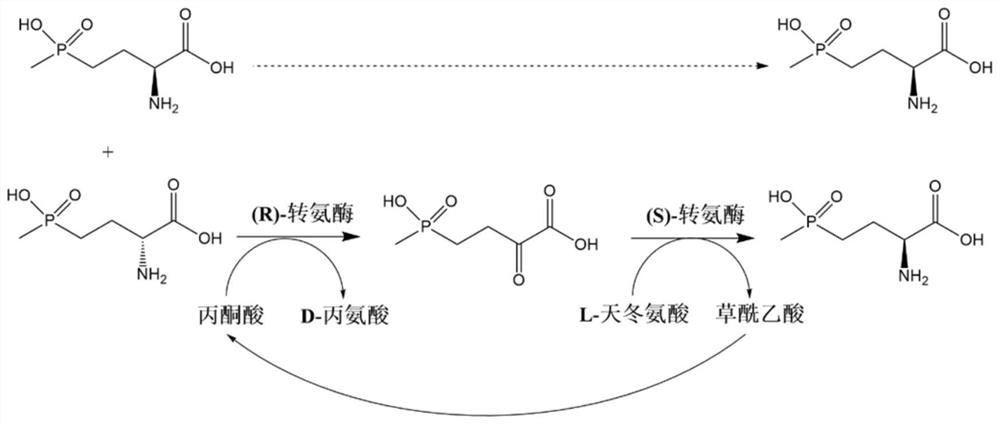
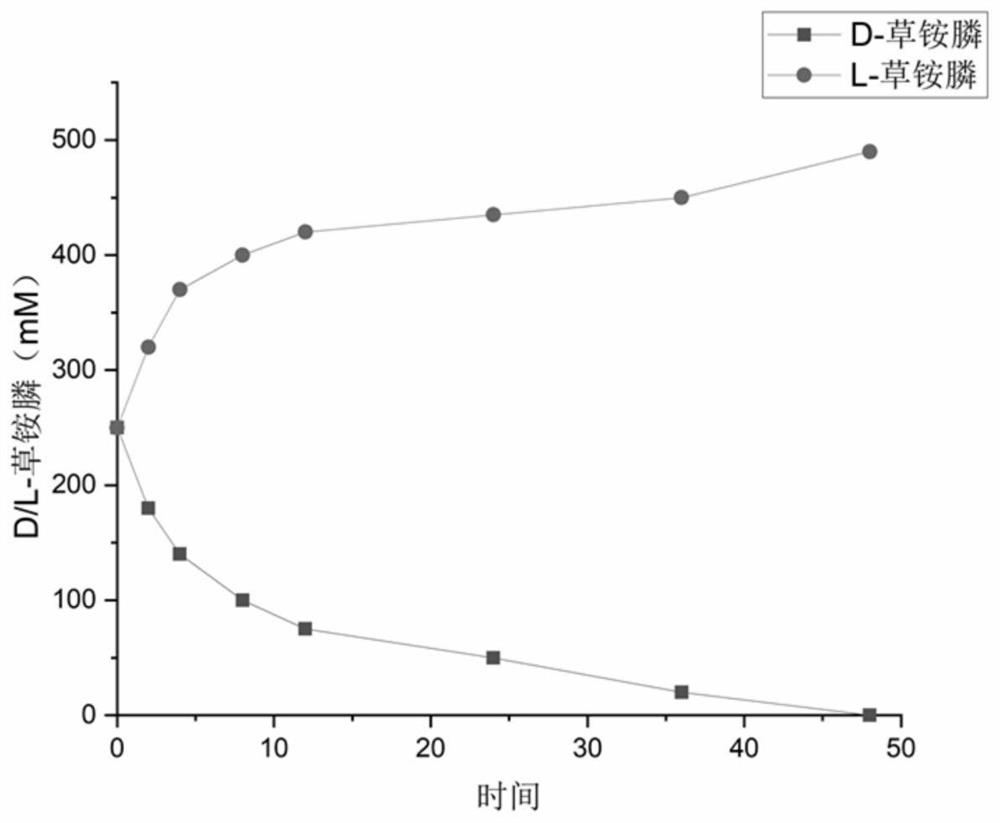
Method for preparing L-glufosinate ammonium by using biological multi-enzyme coupling method
A technology of glufosinate-ammonium and recombinant microorganisms, which is applied in the biological field, can solve the problems of difficult separation and purification of products, difficulty in increasing the dosage of substrates, etc., and achieve the effects of high product yield, low production cost, and simple separation and purification process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1: the cultivation of engineering bacterium thalline
[0049] The engineering bacteria E.coli BL21(DE3) / pCDFduet-1-APH1, E.coli BL21(DE3) / pCDFduet-1-EN5, E.coli BL21(DE3) / pCDFduet-1-APH1-EN5 were streaked on the plate After activation, a single colony was picked and inoculated into 10 mL LB liquid medium containing 50 μg / mL kanamycin, and cultured with shaking at 37°C for 10 h. Transfer 2% of the inoculum into 50 mL of LB liquid medium also containing 50 μg / mL kanamycin, culture with shaking at 37 °C until the OD600 reaches about 0.8, add IPTG with a final concentration of 0.5 mM, and shake at 28 °C Cultivate for 12h. After the cultivation, the culture solution was centrifuged at 8000rpm for 10min, the supernatant was discarded, the bacteria were collected, and stored in a -80°C ultra-low temperature refrigerator until use.
Embodiment 2
[0050] Embodiment 2: Enzyme sequence synthesis and bacterial strain construction
[0051] After the sequence (R)-transaminase (APH1) annotated from Pseudarthrobacter chlorophenolicus (the amino acid sequence is shown in SEQ ID NO.1, the nucleotide sequence is shown in SEQ ID NO.2) after the whole gene synthesis, insert The expression plasmid pET-28a(+) was used to obtain pET28a-APH1. After sequencing verification, pET28a-APH1 was transferred into the expression host E. coli BL21 (DE3) for subsequent expression of the recombinase.
[0052] The sequence derived from Corynebacterium vitaeruminis DSM 20294 annotated as (S)-transaminase (EN5) (the amino acid sequence is shown in SEQ ID NO.3, and the nucleotide sequence is shown in SEQ ID NO.4) After the whole gene synthesis, Insert the expression plasmid pET-28a(+) to obtain pET28a-EN5. After sequencing verification, pET28a-EN3 was transferred into the expression host E. coli BL21 (DE3) for the subsequent expression of the recomb...
Embodiment 3
[0053] Embodiment 3: Contain the construction of the co-expression strain of (R)-transaminase and (S)-transaminase system:
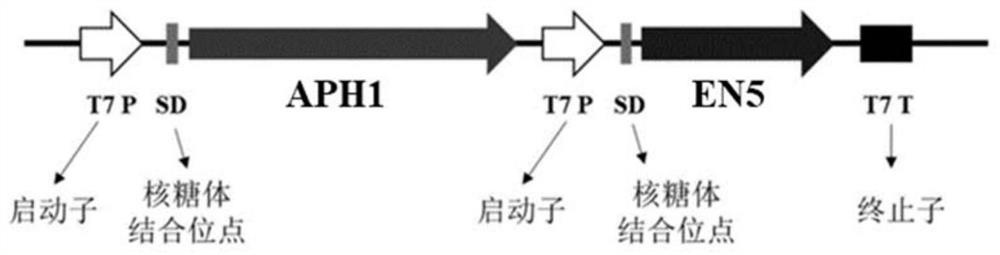
[0054] The APH1 gene used in Example 2 was connected to the multi-cloning site vector pCDFduet-1 by a one-step cloning kit, the restriction sites were HindIII and XhoI, and the one-step cloning primers were C1-F and C1-R (Table 1) , and the plasmid pCDFduet-1-APH1 was constructed. On the basis of the pCDFduet-1-APH1 plasmid, the EN5 used in Example 2 was connected to the second cloning site of the multi-cloning site vector pCDFduet-1 through a one-step cloning kit, and the restriction sites were NdeI and XhoI, the one-step cloning primers are C2-F and C2-R, the plasmid pCDFduet-1-APH1-EN5 is constructed, and the co-expression strain E.coli BL21(DE3) / pCDFduet-1-APH1-EN5 is constructed. APH1-EN5 constructs such as figure 2 shown.
[0055] Table 1: Cloning primer sequences
[0056] Primer sequence C1-F CCCAAGCTTAAGGAGATATACATATGACCTC...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com