Preparation method of culture substrate for batch production of 3D cell spheres
A culture substrate and mass production technology, applied in the field of biomedical material preparation, can solve the problems of expensive 3D cell spheroid culture substrate, large differences in cell spheroid size, unfavorable cell spheroid growth, etc., to improve accuracy and repeatability , the experimental cost reduction, the effect of uniform diameter and structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
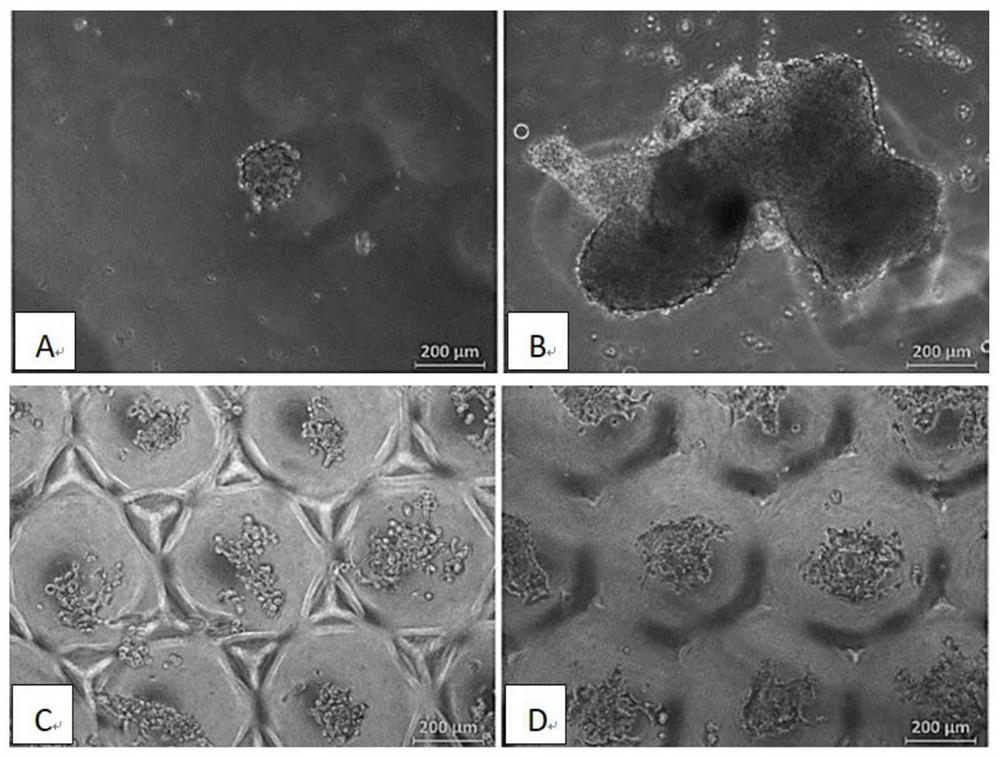
Examples
Embodiment 1
[0027] Effect of Gelatin Concentration
[0028] 1. Obtaining of gelatin microspheres: gelatin particles were dissolved in ultrapure water at 60°C in a water bath, and gelatin solutions with concentrations of 5%, 8%, and 10% (wt / v, g / mL) were prepared respectively. Collect gelatin microspheres with a microfluidic device, by controlling the flow rate of the aqueous phase solution (gelatin solution) to be 5ml / h, the flow rate of the organic phase (based on toluene, adding 3wt% Span80 as a surfactant) flow rate is 40ml / h, and the capillary inner diameter 0.85mm is used as the organic phase channel, and a syringe needle (0.45mm inner diameter) is used as the aqueous phase solution channel inside the capillary to obtain gelatin microspheres, which are collected in methanol solution for subsequent use;
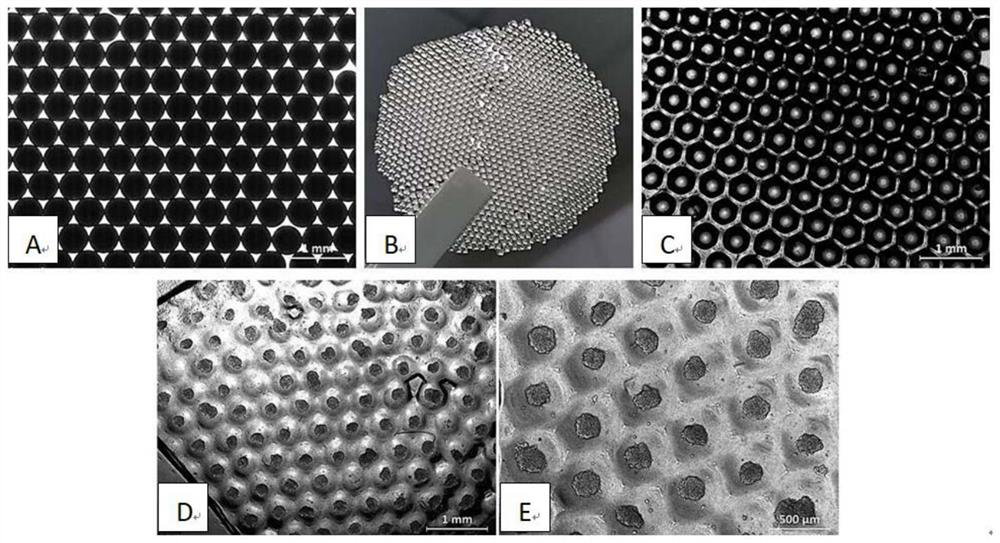
[0029] 2. Preparation of gelatin template: drop gelatin microspheres into a watch glass from the edge, slightly vibrate the watch glass to arrange the gelatin microspheres in a singl...
Embodiment 2
[0036] Influence of Channel Diameter of Aqueous Solution
[0037] This example is basically the same as Example 1, except that the concentration of gelatin is 8%, and the diameters of the channels of the aqueous phase solution are controlled to be 0.3mm and 0.45mm respectively.
[0038] The average diameters of the gelatin microspheres prepared with the channel diameters of 0.3 mm and 0.45 mm in the aqueous phase were 300 μm and 500 μm, respectively. Gelatin microspheres with uniform diameters can be obtained at both flow rates, and the diameters are slightly different. Based on the requirements for subsequent 3D cell sphere culture, the diameter of the aqueous phase channel of 0.45 mm is selected.
Embodiment 3
[0040] Influence of Channel Diameter of Organic Phase
[0041] This example is basically the same as Example 1, except that the gelatin concentration is 8%, and the channel diameters of the organic phase are controlled to be 0.85mm and 1.2mm respectively.
[0042] The average diameters of the gelatin microspheres prepared with organic phase channel diameters of 0.85 mm and 1.2 mm were 500 μm and 900 μm, respectively. It can be seen that increasing the channel diameter of the organic phase can obtain gelatin microspheres with larger sizes, indicating that the present invention can adjust the size of the obtained gelatin microspheres by changing the inner diameters of the organic phase and aqueous phase channels, and further affect the size of the subsequent 3D cell spheres.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com