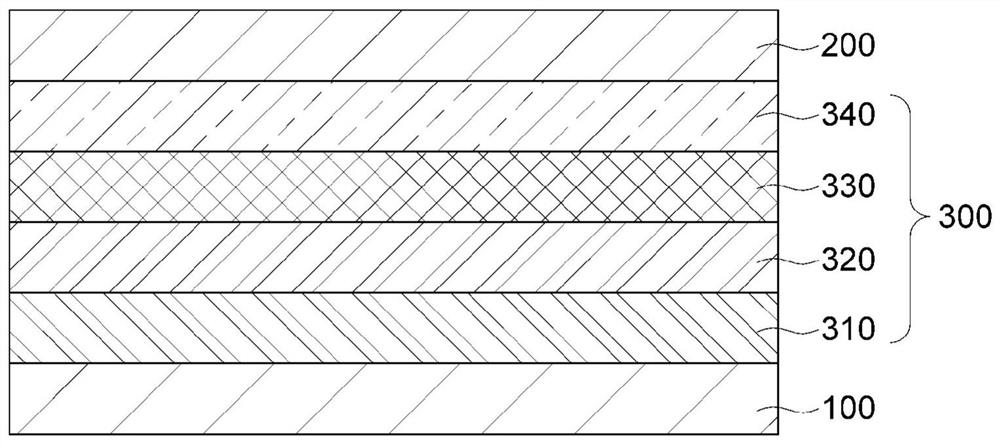
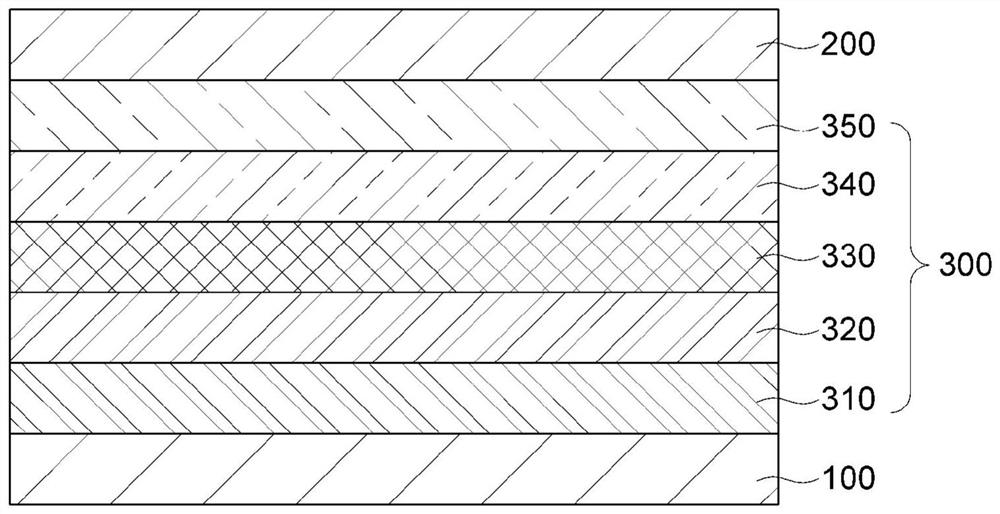
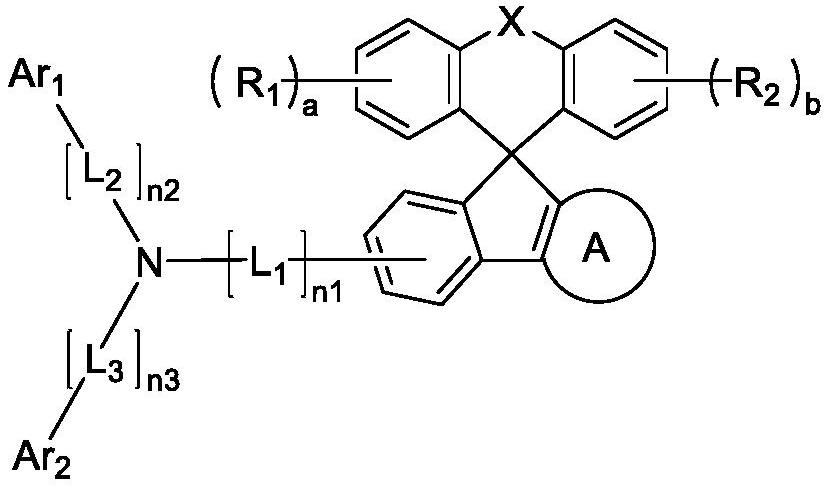
Organic light-emitting compound, and organic electroluminescent device using same
An organic compound and compound technology, applied in the field of organic electroluminescent elements, can solve the problems of poor thermal stability, low glass transition temperature, unsatisfactory life of organic EL elements, etc., and achieve improved efficiency and hole transport capability. excellent effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0217] [Synthesis Example 1] Synthesis of Compound 2
[0218]
[0219] In 10.0g (21.7mmol) of 9-bromospiro[benzo[c]fluorene-7,9'-xanthene] obtained in Preparation Example 1 and 7.0g (21.7mmol) of bis([1,1'- After adding 100 mL of toluene to biphenyl]-4-yl)amine, 1.0 g (1.1 mmol) of Pd was added to the reaction solution 2 (dba) 3 , 1.04g (2.2mmol) of XPhos, and 4.2g (43.4mmol) of NaOt-Bu were heated and refluxed at 120° C. for 3 hours. Then, the temperature of the reaction liquid after the above-mentioned heating and reflux was cooled to normal temperature, and 300 mL of purified water was poured into the reaction liquid to complete the reaction. The mixed liquid obtained after completion of the reaction was extracted with 500 mL of E.A., and washed with distilled water to obtain an organic layer. The resulting organic layer was treated with anhydrous MgSO 4 After drying and distillation under reduced pressure, it was purified by gel column chromatography to obtain 11....
Synthetic example 2
[0221] [Synthesis Example 2] Synthesis of Compound 4
[0222]
[0223] Use N-([1,1'-biphenyl]-4-yl)-[1,1'-biphenyl]-2-amine to replace the bis([1,1'-biphenyl) used in Synthesis Example 1 Except for phenyl]-4-yl)amine, the same procedure as [Synthesis Example 1] was carried out to obtain 9.9 g of the target compound (yield 65%).
[0224] [LCMS]: 701
Synthetic example 3
[0225] [Synthesis Example 3] Synthesis of Compound 7
[0226]
[0227] Use N-([1,1'-biphenyl]-2-yl)-9,9-dimethyl-9H-fluoren-2-amine to replace the bis([1,1' Except for -biphenyl]-4-yl)amine, the same procedure as in [Synthesis Example 1] was carried out to obtain 8.5 g of the target compound (yield 70%).
[0228] [LCMS]: 741
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com