Carbocisteine oral solution and preparation method thereof
A technology of oral solution and carbocisteine, which is applied in the fields of pharmaceutical formulation, dispersion liquid delivery, and medical preparations of non-active ingredients, etc. It can solve problems such as poor product quality, reduction of active ingredients, and unstable long-term storage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] The present invention also provides a kind of preparation method of carbocisteine oral solution, the method comprises the following steps:
[0032] (1) Take water for injection, add sodium hydroxide (the molar ratio to carbocisteine is 0.950~1.025) to dissolve completely, add carbocisteine, stir, dissolve completely, filter the membrane to obtain carbocisteine solution for subsequent use;
[0033] (2) Take water for injection, add methylparaben, stir and dissolve, pass through a sieve, and keep warm for subsequent use;
[0034] (3) Add the carbocisteine solution obtained in step (1) into the solution obtained in step (2), dilute to full volume with water for injection, stir evenly, fill, and melt seal.
[0035] Wherein, when the carbocisteine oral solution contains one or more of glycerin, caramel, and stevioside, add carbocisteine in step (1) and stir to dissolve completely, then add glycerin, caramel, and stevioside One or more of them, then stir evenly, ...
Embodiment 1
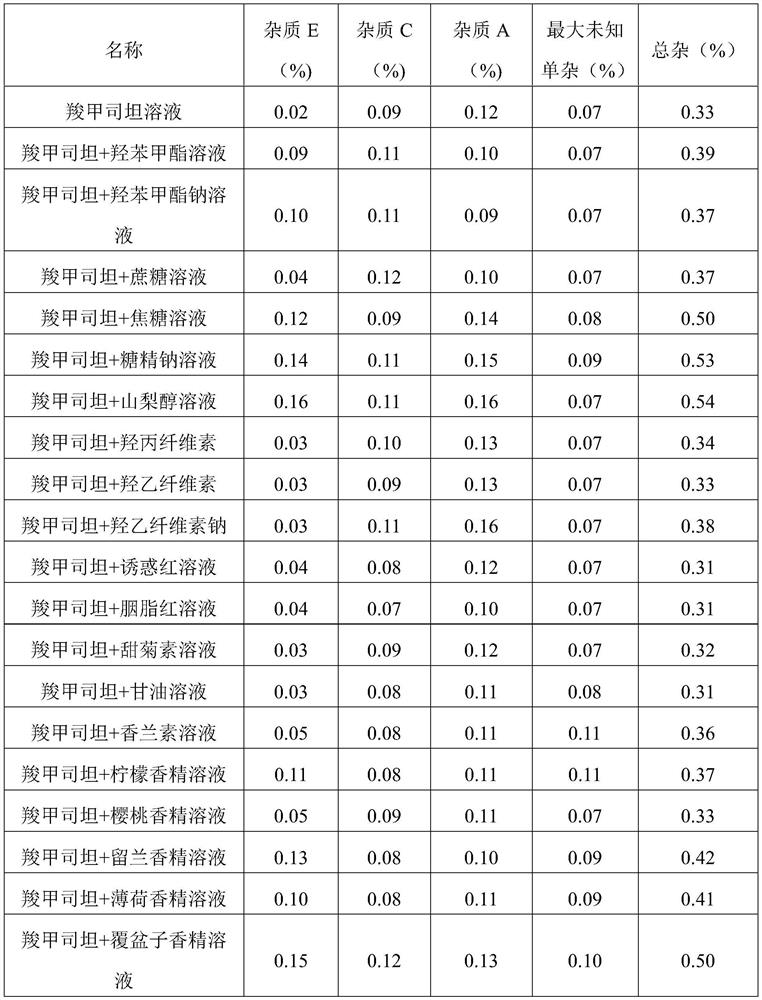
[0048] Embodiment 1 single auxiliary material compatibility investigation
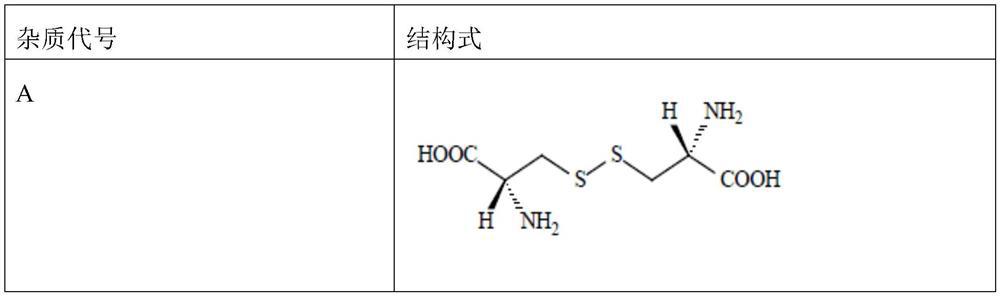
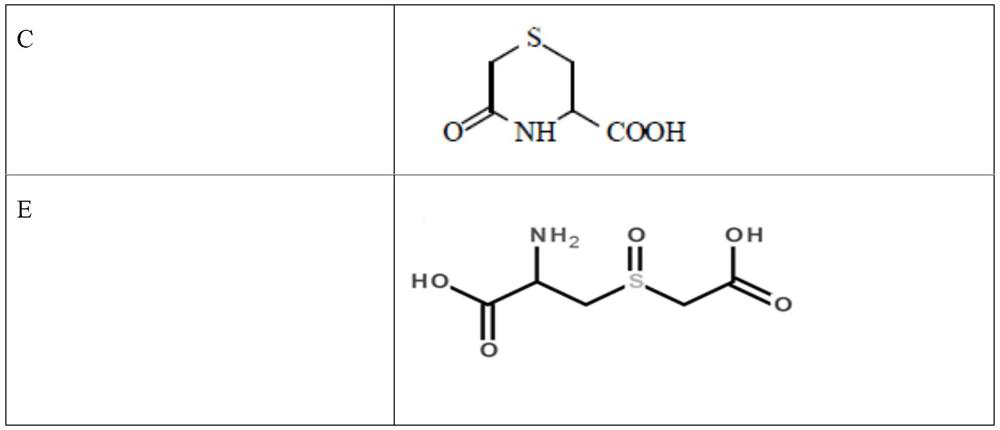
[0049] In this embodiment, investigate carbocisteine or carbocisteine single adjuvant, in the compatibility test of multiple adjuvants, carbocisteine and each adjuvant are added by content shown in table 2, and described antibacterial agent can be It is methylparaben or sodium methylparaben; the syrup base can be caramel, sucrose, sorbitol or sodium saccharin; the flavoring agent can be stevia, glycerin, vanillin, lemon flavor, cherry flavor , spearmint essence, mint essence or raspberry essence, wherein if the corrective agent selects glycerin, the addition amount of glycerin is 1 gram, and in addition, the addition amount of other corrective agents is 0.1 gram.
[0050] Table 2 Addition amount of each component in the compatibility test of excipients
[0051] Element Amount added / g carbocisteine 5 antibacterial agent 0.15 Hydroxypropyl Cellulose / Ethyl Cellulose / Sodi...
Embodiment 2
[0068] Embodiment 2: investigation of full prescription compatibility
[0069] According to the test results of Example 1, several prescriptions were selected, and the full prescription compatibility test was carried out according to the addition amount in Table 2. The specific prescriptions and test results are shown in Table 6-8.
[0070] Table 6 full prescription compatibility test (0 days)
[0071]
[0072]
[0073] Table 7 Full prescription compatibility test (30 days at 40°C)
[0074]
[0075] Table 8 full prescription compatibility test (light for 30 days)
[0076]
[0077]
[0078] It is not difficult to find out from the above results that the compatibility of each prescription auxiliary material shown is good with the raw material, especially compounded with carbocisteine, methyl paraben, sucrose, allura red and cherry essence, or carbocisteine, The compound of methyl paraben, sucrose and caramel, or the compound of carbocisteine, methyl paraben, suc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com