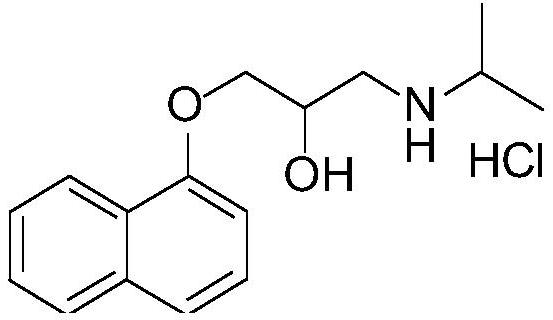
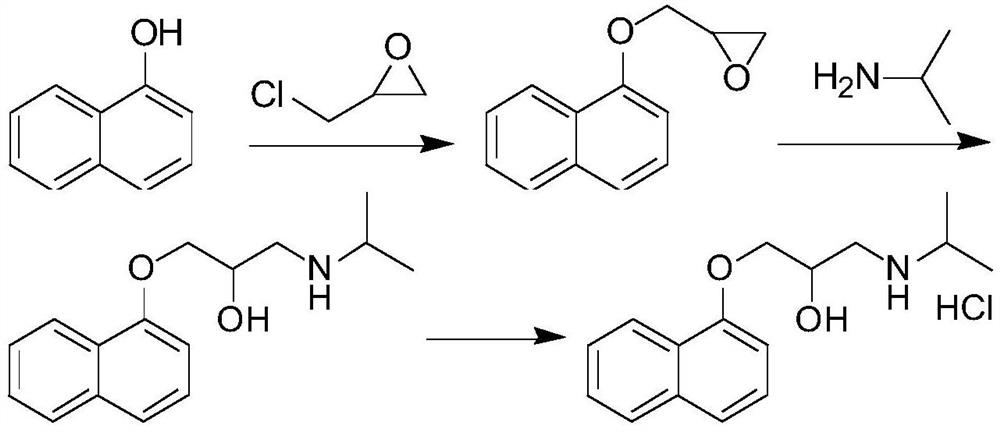
Purification method for propranolol hydrochloride key intermediate
A technology of propranolol hydrochloride and a purification method is applied in the field of purification of key intermediates of propranolol hydrochloride, and can solve the problems of low purity and yield, strict requirements for purification equipment, large post-treatment pollution, etc. Simple, overcoming stringent requirements and high boiling pollution problems, the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
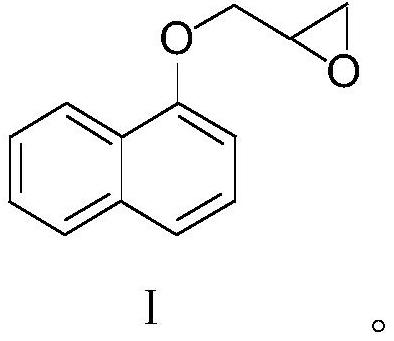
[0034] Weigh 1-naphthol (144.1g, 1.0mol, 1.0eq), N,N-diisopropylethylamine (6.5g, 0.05mol, 0.05eq), epichlorohydrin (277.5g, 3.0mol, 3.0 eq) was added to a 1L four-necked flask, stirred and heated to 95°C for reflux reaction, and TLC monitored the reaction end point (V PE / EA=5:1). After the reaction reached the end point, the temperature was lowered to 50°C, and 30wt% NaOH aqueous solution (200g , 1.5mol, 1.5eq), quenched the reaction, the reaction solution was down to room temperature, 500ml ethyl acetate was added thereto, stirred for 5min, the solution was layered and separated, the organic phase was washed 2 times with 200ml of water respectively, and the organic phase was washed for 35 Concentrate to dryness at ~45°C under reduced pressure to obtain 215.3 g of the crude key intermediate, and load the crude key intermediate into a silica gel chromatography column (the weight of the silica gel packed in the chromatography column is 2.5 kg), using a volume ratio of PE / EA=17: ...
Embodiment 2
[0036] Weigh 1-naphthol (144.1g, 1.0mol, 1.0eq), N,N-diisopropylethylamine (6.5g, 0.05mol, 0.05eq), epichlorohydrin (277.5g, 3.0mol, 3.0 eq) was added into a 1L four-neck flask, stirred and raised to 95°C to reflux for reaction, and the reaction endpoint was monitored by TLC (V PE / EA=5:1).
[0037] After the reaction reached the end point, the temperature was lowered to 50°C, and 30wt% NaOH aqueous solution (200g, 1.5mol, 1.5eq) was added dropwise to quench the reaction. The layers were separated, the organic phase was washed twice with 200ml water respectively, and the organic phase was concentrated to dryness under reduced pressure at 35~45°C to obtain 208.1g of the crude product of the key intermediate, and the crude product of the key intermediate was loaded into a silica gel chromatography column (chromatographic column The weight of loaded silica gel is 2.0kg), the small polar impurities are eluted with a mixed solvent with a volume ratio of PE / EA=15:1, TLC monitors the ...
Embodiment 3
[0039]Weigh 1-naphthol (144.1g, 1.0mol, 1.0eq), N,N-diisopropylethylamine (6.5g, 0.05mol, 0.05eq), epichlorohydrin (277.5g, 3.0mol, 3.0 eq) was added into a 1L four-neck flask, stirred and raised to 95°C to reflux for reaction, and the reaction endpoint was monitored by TLC (V PE / EA=5:1).
[0040] After the reaction reached the end point, the temperature was lowered to 50°C, and 30wt% NaOH aqueous solution (200g, 1.5mol, 1.5eq) was added dropwise to quench the reaction. The layers were separated, the organic phase was washed twice with 200ml of water, and the organic phase was concentrated to dryness under reduced pressure at 35 to 45°C to obtain 210.0g of the crude product of the key intermediate, and the crude product of the key intermediate was loaded into a silica gel chromatography column (chromatographic column packing The weight of the silica gel is 1.5kg), and the small polar impurities are eluted with a mixed solvent with a volume ratio of PE / EA=18:1. TLC monitors the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com