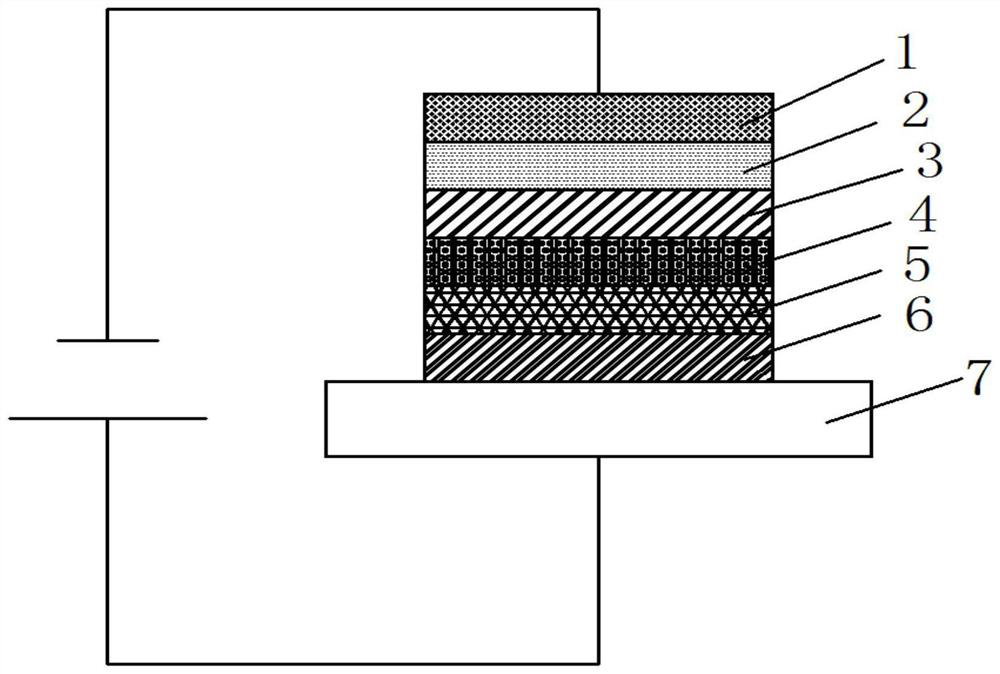
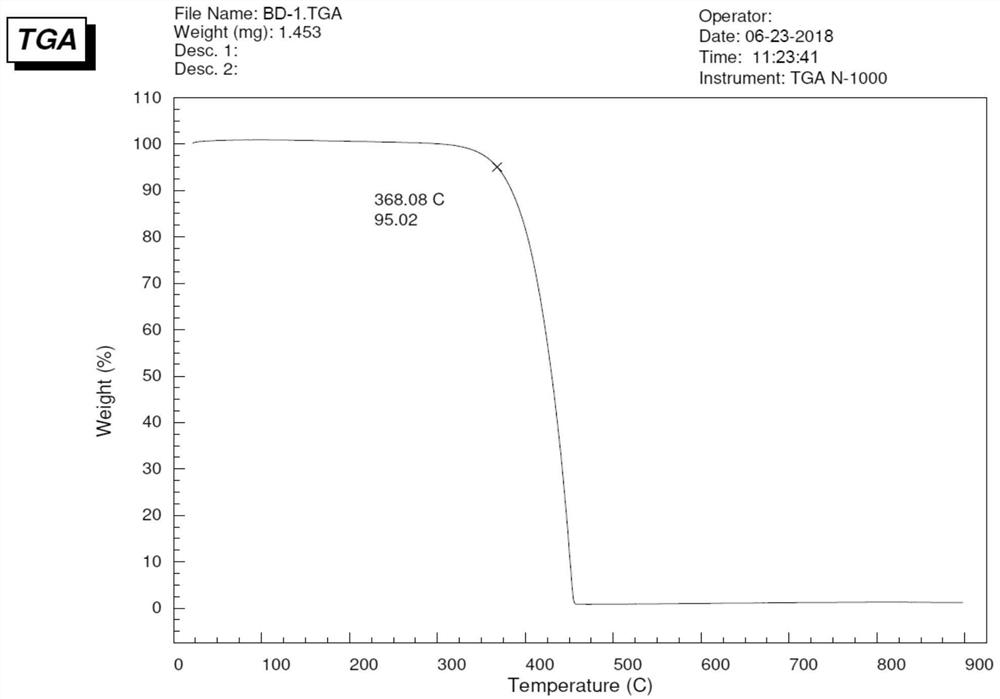
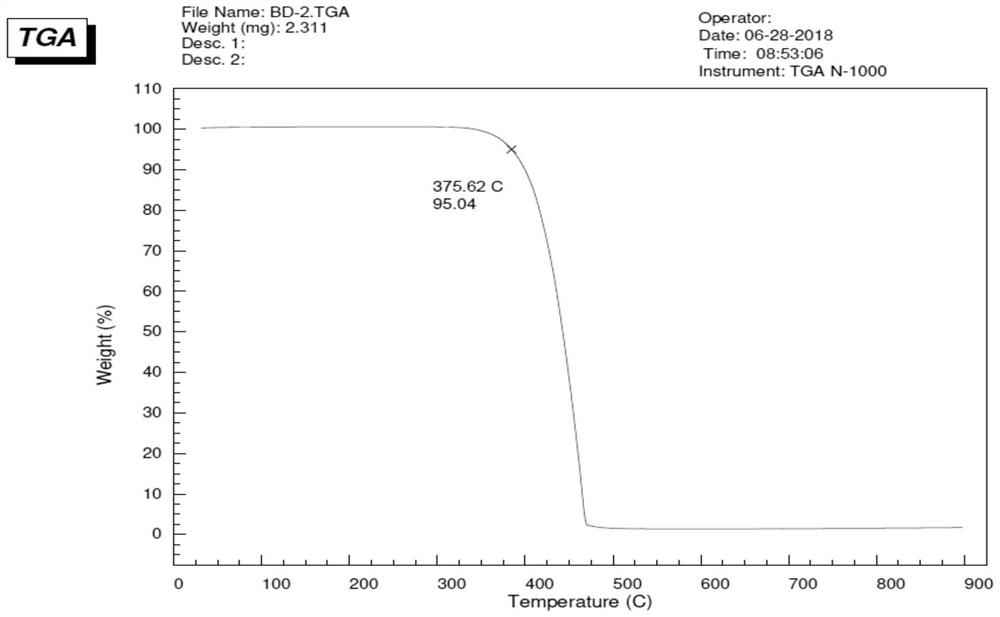
A blue fluorescent doped material and organic electroluminescent device with good thermal stability and high efficiency
A technology of blue fluorescent and doped materials, which is applied in the field of blue fluorescent doped materials and organic electroluminescent devices, which can solve the problems of reducing the working stability of materials, poor matching of electron orbital energy levels, and difficulties in light-emitting devices. , to achieve the effects of improving thermal stability and luminous efficiency, good conjugation effect, and improving luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037]
[0038] The synthesis method of the blue fluorescent doping material (1) is as follows:
(1)
[0039]
[0040] Under nitrogen protection, compound 1-a (5.0g, 438.95g / mol, 11.39mmol), compound 1-b (1eq, 2.32g, 203.94g / mol, 11.39mmol), sodium tert-butoxide (1.1eq, 1.2 g, 96.1g / mol, 12.53mmol), tris(dibenzylideneacetone)dipalladium (0.05eq, 0.52g, 915g / mol, 0.57mmol), tri-tert-butylphosphine (0.05eq, 0.12g, 202.32g) / mol, 0.57mol) and toluene (50ml) were added to the reaction flask, the temperature was raised to reflux for 5h after the addition of materials, and after the reaction was completed, the temperature was lowered to room temperature, 50ml of water was added and stirred for 15min to obtain a filtrate. The filtrate was filtered through diatomaceous earth. The organic phase was obtained by liquid separation. The organic phase was dried with anhydrous magnesium sulfate and then spin-dried. After purification by column chromatography, compound 1-c (4.72 g, yie...
Embodiment 2
[0046]
[0047] The synthesis method of the blue fluorescent doping material (6) is as follows:
[0048] Step (1) is the same as Example 1
(2)
[0049]
[0050] Under nitrogen protection, compound 2-a (2g, 514.98g / mol, 3.88mmol), compound 2-b (1eq, 0.87g, 225.15g / mol, 3.88mmol), sodium tert-butoxide (1.1eq, 0.41g) were combined , 96.1g / mol, 4.27mmol), tris(dibenzylideneacetone)dipalladium (0.05eq, 0.178g, 915g / mol, 0.19mmol), tri-tert-butylphosphine (0.05eq, 0.039g, 202.32g / mol, 0.19mol) and toluene (20ml) were added to the reaction flask, the temperature was raised to reflux for 5h after the addition of materials, and after the reaction was completed, the temperature was lowered to room temperature, and 20ml of water was added to stir for 15min to obtain a filtrate. The organic phase was obtained from the liquid. The organic phase was dried over anhydrous magnesium sulfate and then spin-dried. After purification by column chromatography, compound 2-c (1.94 g, yield ...
Embodiment 3
[0054]
[0055] The synthesis method of the blue fluorescent doping material (11) is as follows:
[0056] Step (1) is the same as Example 1
(2)
[0057]
[0058] Under nitrogen protection, compound 3-a (2g, 514.98g / mol, 3.88mmol), compound 3-b (1eq, 1.54g, 397.18g / mol, 3.88mmol), sodium tert-butoxide (1.1eq, 0.41g) , 96.1g / mol, 4.27mmol), tris(dibenzylideneacetone)dipalladium (0.05eq, 0.178g, 915g / mol, 0.19mmol), tri-tert-butylphosphine (0.05eq, 0.039g, 202.32g / mol, 0.19mol) and toluene (20ml) were added to the reaction flask, the temperature was raised to reflux for 5h after the addition of materials, and after the reaction was completed, the temperature was lowered to room temperature, and 20ml of water was added to stir for 15min to obtain a filtrate. The organic phase was obtained from the liquid. The organic phase was dried with anhydrous magnesium sulfate and then spin-dried. After purification by column chromatography, compound 3-c (2.3 g, yield 71.2%) was obt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com