Liver-targeted peroxynitrite fluorescent probe as well as preparation method and application thereof
A technology of peroxynitrite and fluorescent probes, applied in the fields of fluorescence/phosphorescence, chemical instruments and methods, and material analysis through optical means, which can solve the problems that cannot be studied in depth, probes lack tissue or organ targeting, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
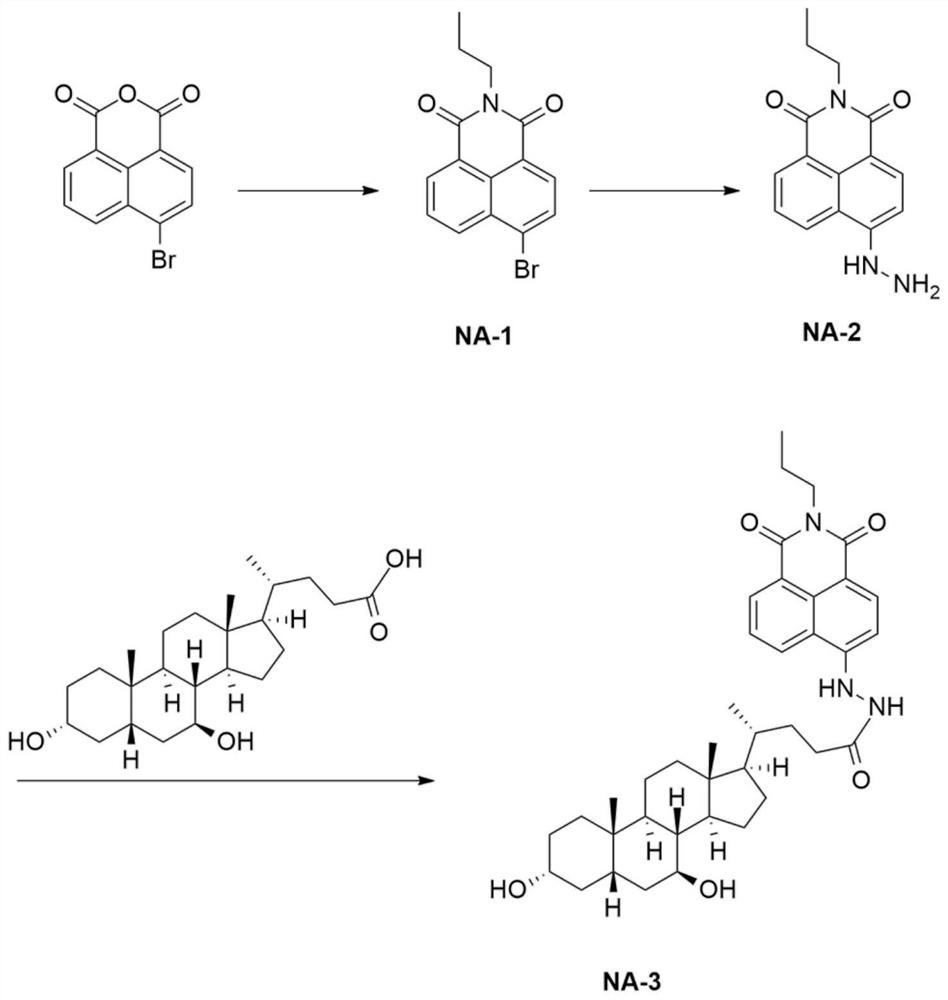
[0036] A liver-targeted peroxynitrite fluorescent probe
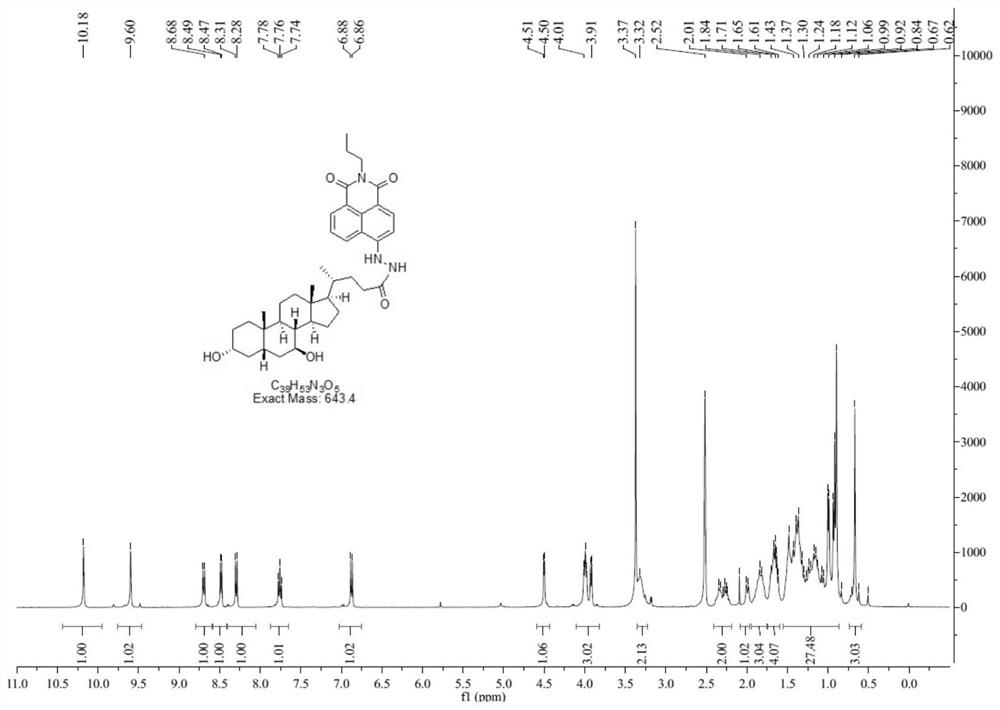
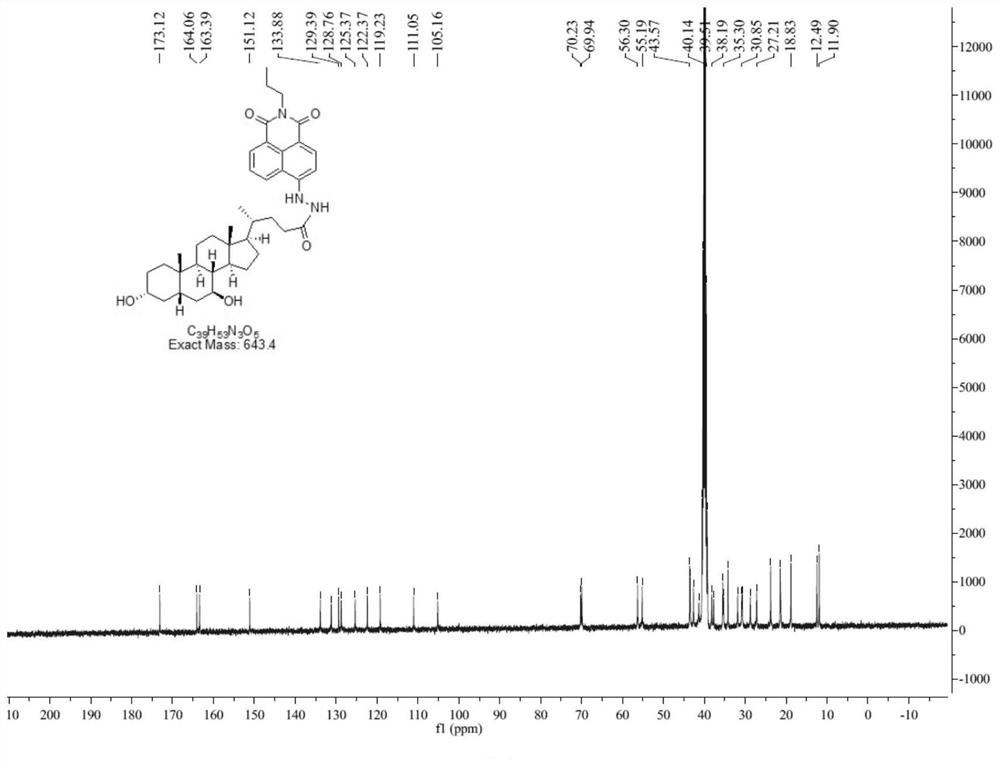
[0037] (1) Synthesis of compound NA-1: Compound 4-bromo-1,8-naphthalic anhydride (5g, 18.05mmol) was dissolved in 150mL of dioxane, stirred until clear, and n-propylamine (1.12g, 19.85 mmol), replaced with nitrogen, and reacted at 110° C. for 8 hours. TLC showed that the reaction was complete, the solvent was spin-dried, and the crude product was separated by column chromatography (petroleum ether: ethyl acetate = 40:1-10:1) to obtain 4 g of a yellow solid. 1 H NMR (400MHz, DMSO-d 6 )δ8.55-8.50 (m, 2H), 8.31 (d, J = 8.0Hz, 1H), 8.31 (d, J = 8.0Hz, 1H), 8.20 (d, J = 8.0Hz, 1H), 7.99 ( t,J=12.0Hz,1H), 4.01(t,J=16.0Hz,2H), 1.69-1.67(m,2H), 0.95(t,J=12.0Hz,3H); 13 C NMR (100MHz, DMSO-d 6 )δ163.1, 132.9, 131.9, 131.7, 131.2, 130.0, 129.5, 128.5, 123.0, 122.2. HRMS: [M+H + ] 318.0131, Calculated: 318.0124;
[0038] (2) Compound NA-1 (2g, 6.29mmol) was dissolved in 50mL of 2-methoxyethanol, 85% hydrazine hydrate (1mL, 12...
Embodiment 2
[0041] Fluorescent probes for different concentrations of ONOO - identification
[0042] Dissolve the fluorescent probe obtained in Example 1 with dimethyl sulfoxide, prepare the probe mother solution, and add different equivalents of ONOO - The solution was incubated at 37° C. for 30 min, diluted with phosphate buffer solution to a concentration of 10 μM to be tested, and then measured by fluorescence spectrum. Such as Image 6 As shown, the probe was able to respond to 0-14 μM ONOO - Response, and has a good linear relationship at 550nm, the linear regression equation is y=7.48x+0.24, R 2 = 0.9933.
Embodiment 3
[0044] Fluorescent probes are affected by the pH environment
[0045] Dissolve the fluorescent probe obtained in Example 1 with dimethyl sulfoxide, prepare the probe mother solution, and add 10 equivalents of ONOO - , after incubating at 37°C for 30min, dilute it to the test concentration of 10μM with phosphate buffer solution with different pH values, and measure its fluorescence spectrum (λ ex =450nm); according to its fluorescence intensity, evaluate the impact of different pH environments on the fluorescence intensity of the fluorescent probe, the results are as follows Figure 7 shown. The pH values are 4, 5, 6, 7, 8, 9, respectively. Fluorescent probes with ONOO - The fluorescence intensity before and after the treatment was not affected by the pH environment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com