Method for preparing hydrotalcite
A technology of aluminum magnesium carbonate and mixed alkali solution, applied in chemical instruments and methods, aluminum compounds, inorganic chemistry, etc., can solve the problems of short anti-acid time, influence of acid-making power, inability to achieve washing, etc., to achieve excellent performance and operation. Simple and convenient effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1 prepares hydromagnesium carbonate with the feeding mixed alkali solution of the feeding mode that disperses and drops
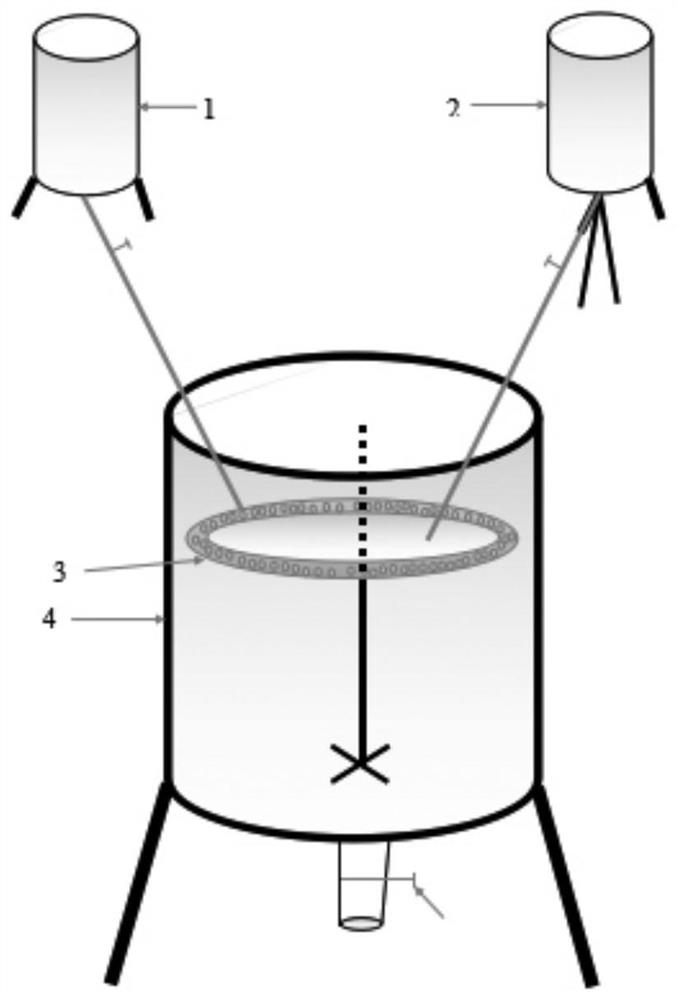
[0034] The molar ratio of materials is: nAl 3+ : mMg 2+ :xOH - :yCO 3 2- =1:3:9:1.0, the pH of the reaction system is controlled to be 10-11 during the dropping process. The schematic diagram of the reaction equipment is shown in figure 1 shown.
[0035] Preparation Process:
[0036] Preparation of mixed salt solution: Dissolve 150.4kg of magnesium chloride and 70.4kg of aluminum chloride into 200kg of water, stir and dissolve to form a mixed salt solution;
[0037] Preparation of mixed alkali solution: dissolve 56.2kg of sodium carbonate and 190.8kg of sodium hydroxide into 200kg of water, stir and dissolve to form a mixed alkali solution;
[0038] The prepared mixed alkali solution is dispersed and dropped into the reactor at a speed of 0.9kg / min, and the prepared mixed salt solution is added dropwise to the reactor according to ...
Embodiment 2
[0042] Embodiment 2 prepares aluminum magnesium carbonate by feeding and mixing alkali solution in the feeding mode of dispersing and dripping
[0043] The molar ratio of materials is: nAl 3+ : mMg 2+ :xOH-:yCO 3 2- = 1: 3: 14: 1.5, the pH of the reaction system is controlled during the dropping process = 11-12. The schematic diagram of the reaction equipment is shown in figure 1 shown.
[0044] Preparation Process:
[0045] Preparation of mixed salt solution: Dissolve 117.3kg of magnesium chloride and 70.4kg of aluminum sulfate in 200kg of water together to form a mixed salt solution;
[0046] Prepare mixed alkali solution: dissolve 44.5kg sodium carbonate and 151.2kg sodium hydroxide into 200kg water together to form mixed alkali solution;
[0047] Then the mixed alkali solution is dispersed and dripped into the reactor at a speed of 2.5kg / min, and the mixed salt solution prepared is added dropwise to the reactor in a conventional (single-point drip) manner to react, ...
Embodiment 3
[0050] Embodiment 3 prepares aluminum magnesium carbonate by feeding and mixing alkali solution in the feeding mode of dispersing and dripping
[0051] The molar ratio of materials is: nAl 3+ : mMg 2+ :xOH-:yCO 3 2- =1:3:12:1.2, the pH of the reaction system is controlled to be 10-11 during the dropping process.
[0052] Preparation Process:
[0053] Preparation of mixed salt solution: dissolve 191.4kg of magnesium sulfate and 70.4kg of aluminum chloride into 200kg of water to form a mixed salt solution;
[0054] Preparation of mixed alkali solution: dissolve 67.4kg of sodium carbonate and 254.4kg of sodium hydroxide into 200kg of water together to form a mixed alkali solution;
[0055] Then the mixed alkali solution is dispersed and dripped into the reactor at a speed of 1.6kg / min, and the mixed salt solution prepared is added dropwise to the reactor in a conventional (single-point drip) manner to react, and the whole process is controlled. The pH of the addition proces...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com