Method for quantifying protein abundance by taking metal cluster as artificial antibody
A technology of artificial antibodies and metal clusters, applied in the detection and quantification of low-abundance proteins, can solve the problem of not being able to obtain the exact content of the target protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
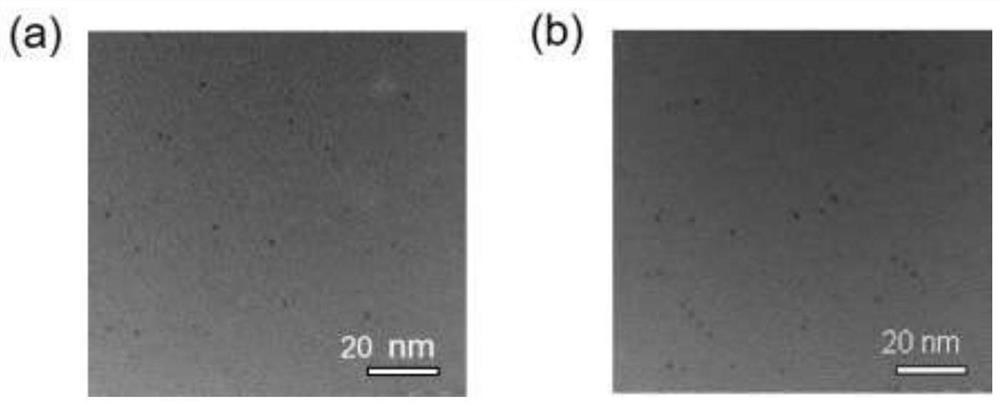
[0040] Example 1: Synthetic polypeptide-protected gold clusters as artificial antibodies
[0041] In this example, the targeting peptide sequences of MMP14 are H 2 N-CHWKHLHNTKTFL-COOH (SEQ ID NO. 1), and H 2 N-HWKHLHNTKTFLC-COOH (SEQ ID NO. 2).
[0042] During the synthesis process, firstly, 5mg of polypeptide (SEQ ID NO.1) was fully dissolved in 1.45mL of ultrapure water to make an aqueous solution of polypeptide, and mixed with 0.117mL of 25mM HAuCl at room temperature 4 Stir evenly, and add 0.24 mL of NaOH solution with a concentration of 0.5 M dropwise under sufficient stirring conditions to adjust the pH value to 12. The system was kept at 37°C for 24 hours, and then centrifuged at 3700 rpm for 10 min to remove large particles with a molecular weight cut-off of 30 kDa, and the supernatant was transferred to an ultrafiltration tube with a molecular weight cut-off of 3 kDa to continue concentrating Gold clusters, so far the preparation of gold cluster 1 is completed. S...
Embodiment 2
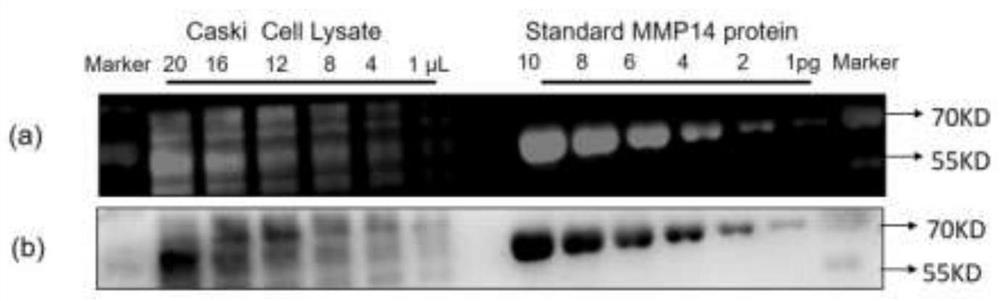
[0043] Example 2: Quantitative detection of MMP14 abundance in protein lysates using gold cluster 1 as an artificial antibody
[0044] Using the gold cluster 1 prepared in Example 1 as an artificial antibody to quantitatively detect MMP14 in the protein lysate of the human cervical cancer cell line Caski cell mainly includes the following contents:
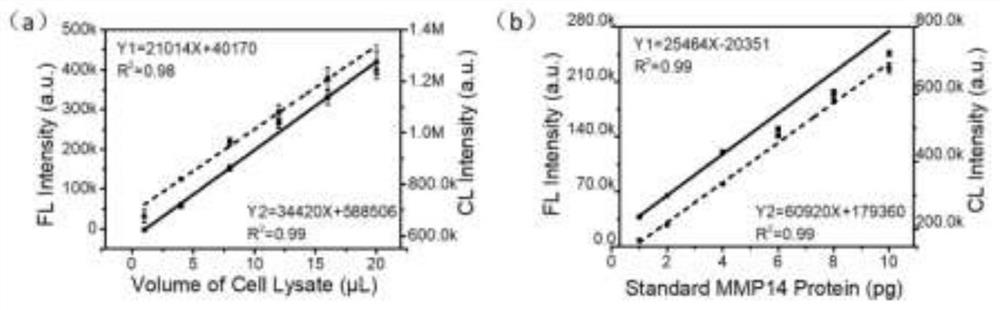
[0045] 1) Separation of standard protein and sample protein by polyacrylamide gel electrophoresis
[0046] Prepare two 1.5mm, 15-well 10% polyacrylamide gels. Add 3 μL of pre-stained marker and 17 μL of loading buffer solution to the loading wells at the extreme ends of each gel, and add 1, 2, 4, 6, 8, 10 pg of standard MMP14 protein in sequence next to the loading wells with markers ( MMP14 standard protein was obtained from Boster Biotechnology Company with a purity of >95%). These six sample wells are used as standard control wells, and a blank well is reserved between the sample wells and the standard wells, and only 20 μL o...
Embodiment 3
[0058] Example 3: Quantitative detection of MMP14 abundance in protein lysates using gold cluster 2 as an artificial antibody
[0059] Using the gold cluster 2 prepared in Example 1 as an artificial antibody to quantitatively detect MMP14 in the human cervical cancer cell line Hela cell protein lysate mainly includes the following contents:
[0060] 1) Separation of standard protein and sample protein by polyacrylamide gel electrophoresis
[0061] Similar to the sample addition method in Example 2. Prepare two 1.5mm, 15-well 10% polyacrylamide gels. For each gel, add 3 μL of pre-stained marker and 17 μL of 1× loading buffer solution to the loading well at the extreme end, and add 0.5, 1, 2, 3, 4, 5 pg of standard MMP14 once to the loading well adjacent to the marker Protein (MMP14 standard protein is from Boster Biotechnology Company, purity>95%). These six sample wells are used as standard control wells, and a blank well is reserved between the sample wells and the standar...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com