Construction of intravenous-injection self-assembled nano-particles of triglyceride prodrug
A technology of nanoparticles and prodrugs, which is applied in drug combinations, antineoplastic drugs, pharmaceutical formulations, etc., can solve problems such as side effects, achieve high-efficiency entrapment, good stability, and simple and easy preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
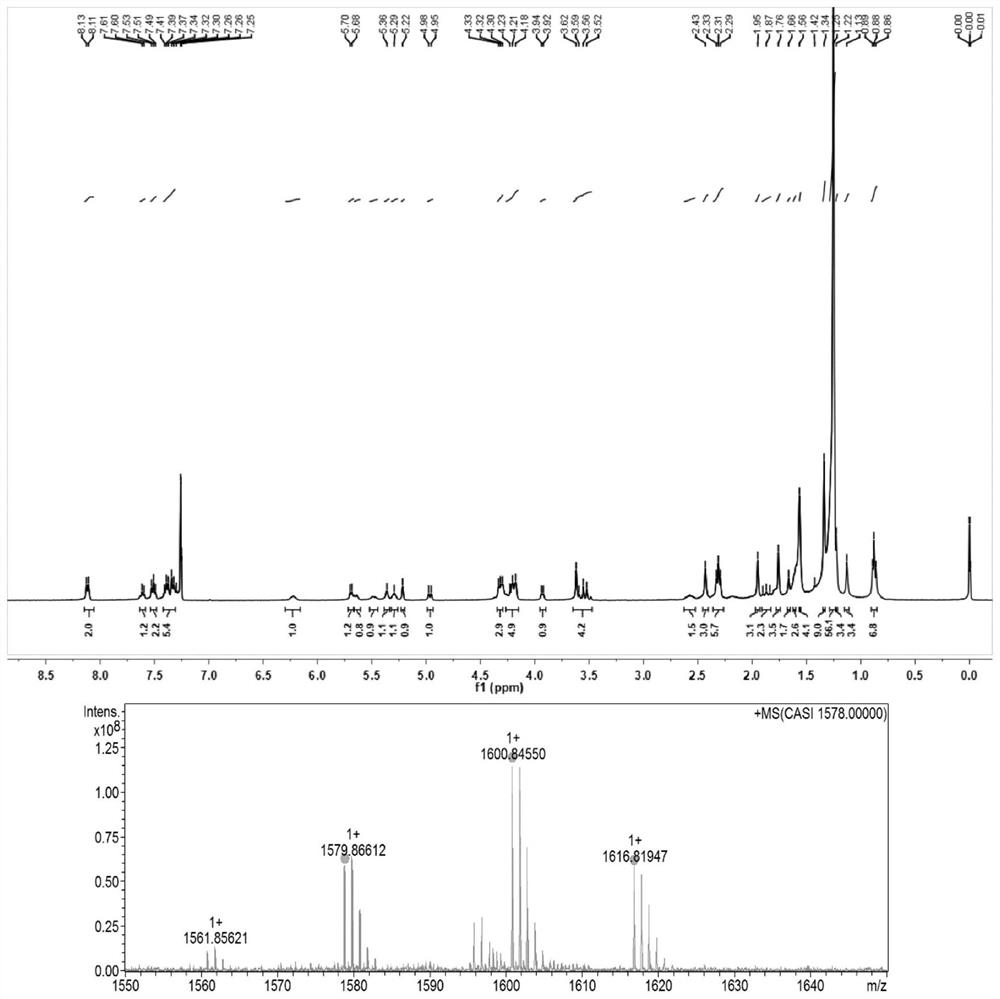
[0031] Example 1: Synthesis of docetaxel-like triglyceride prodrug (DSSTG(0))
[0032] Weigh an appropriate amount of stearic acid, EDCI, and DMAP in a 100ml eggplant-shaped bottle, add 60ml of anhydrous dichloromethane to dissolve and activate it in an ice bath for half an hour, weigh an appropriate amount of 1,3-dihydroxyacetone and add it to the above reaction solution , under the protection of nitrogen, react at room temperature for 48h. After the reaction solution was spin-dried, the crude product was separated by silica gel column chromatography to obtain an intermediate product. Weigh an appropriate amount of the intermediate product and dissolve it in a mixed solvent of tetrahydrofuran and benzene, slowly add 2ml of deionized water to the syringe under stirring, slowly add an appropriate amount of sodium borohydride in an ice bath and continue the reaction for 30 minutes, and slowly add 0.5ml of acetic acid dropwise to terminate the reaction. Add 60ml of chloroform to t...
Embodiment 2
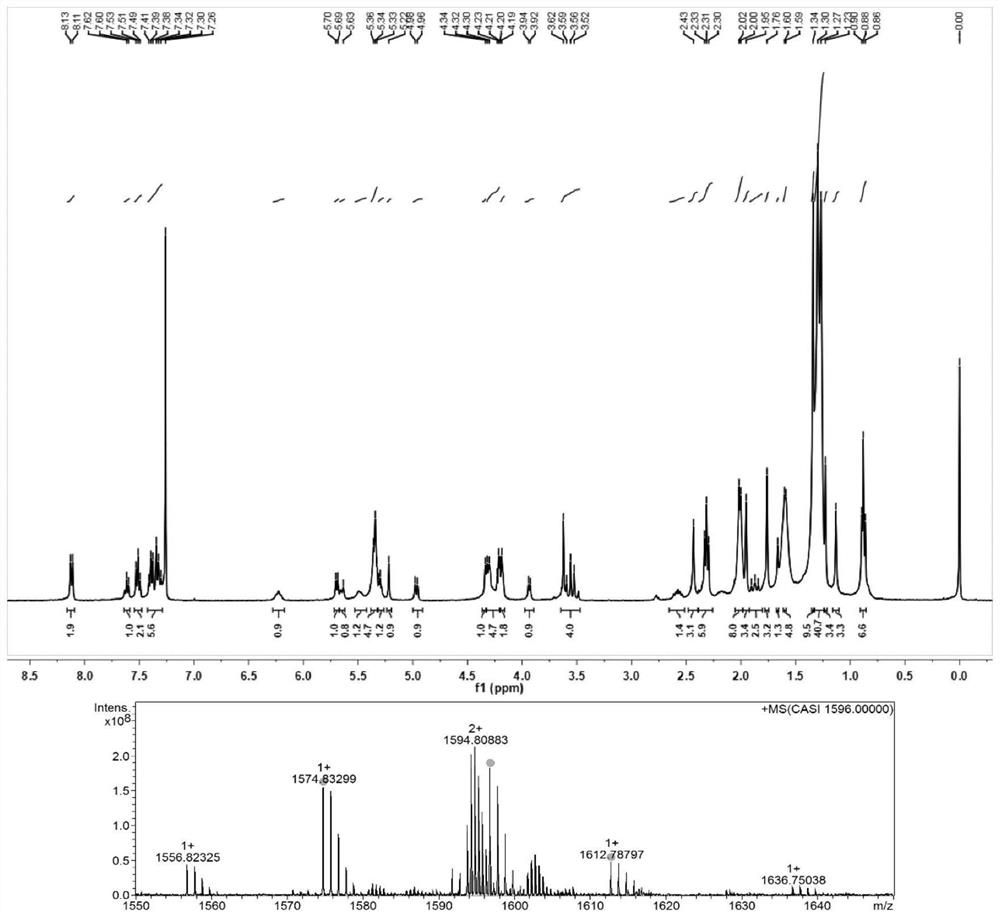
[0035] Example 2: Synthesis of docetaxel-like triglyceride prodrug (DSSTG(1))
[0036] Weigh an appropriate amount of oleic acid, EDCI, and DMAP into a 100ml eggplant-shaped bottle, add 60ml of anhydrous dichloromethane to dissolve and activate it in an ice bath for half an hour, weigh an appropriate amount of 1,3-dihydroxyacetone and add it to the above reaction solution , Under the protection of nitrogen, react at room temperature for 48h. After the reaction solution was spin-dried, the crude product was separated by silica gel column chromatography to obtain an intermediate product. Weigh an appropriate amount of the intermediate product and dissolve it in a mixed solvent of tetrahydrofuran and benzene, slowly add 2ml of deionized water to the syringe under stirring, slowly add an appropriate amount of sodium borohydride in an ice bath and continue the reaction for 30 minutes, and slowly add 0.5ml of acetic acid dropwise to terminate the reaction. Add 60 ml of chloroform t...
Embodiment 3
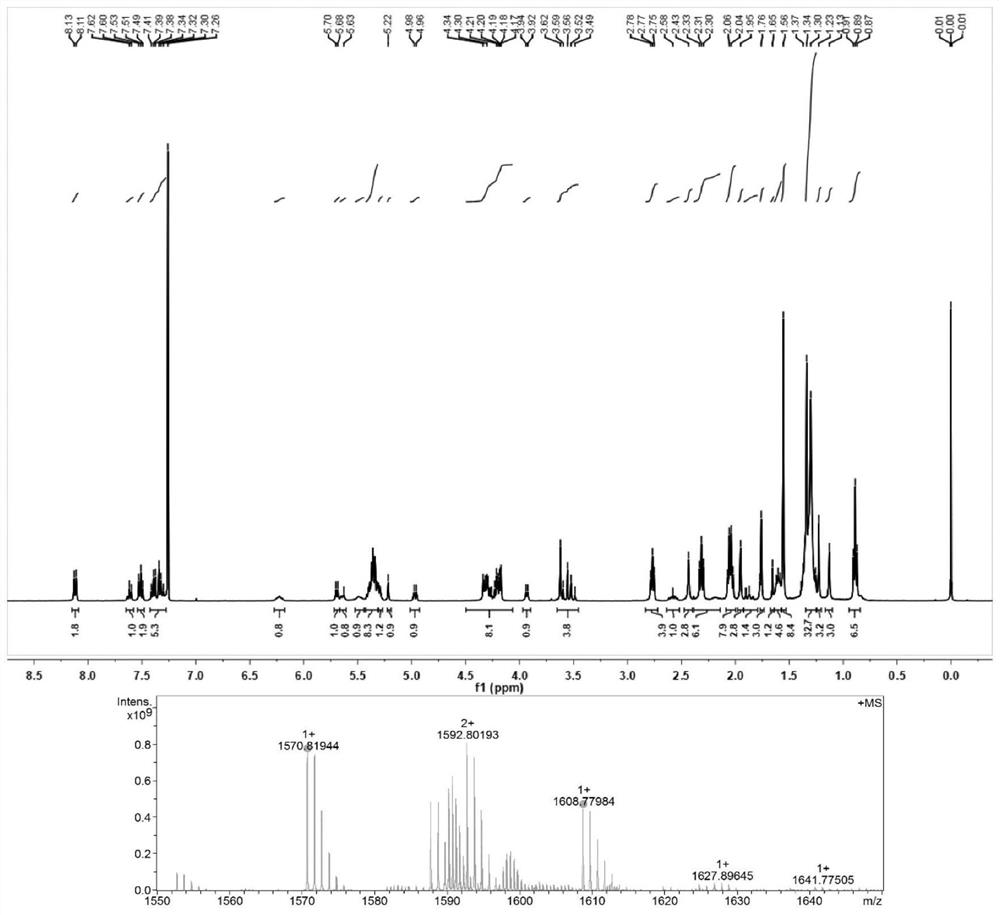
[0039] Example 3: Synthesis of docetaxel-like triglyceride prodrug (DSSTG(2))
[0040] Weigh an appropriate amount of linoleic acid, EDCI, and DMAP into a 100ml eggplant-shaped bottle, add 60ml of anhydrous dichloromethane to dissolve and activate it in an ice bath for half an hour, weigh an appropriate amount of 1,3-dihydroxyacetone and add it to the above reaction solution , under the protection of nitrogen, react at room temperature for 48h. After the reaction solution was spin-dried, the crude product was separated by silica gel column chromatography to obtain an intermediate product. Weigh an appropriate amount of the intermediate product and dissolve it in a mixed solvent of tetrahydrofuran and benzene, slowly add 2ml of deionized water to the syringe under stirring, slowly add an appropriate amount of sodium borohydride in an ice bath and continue the reaction for 30 minutes, and slowly add 0.5ml of acetic acid dropwise to terminate the reaction. Add 60 ml of chlorofor...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com