Continuous preparation method of trifluoroacetyl fluoride
A technology of trifluoroacetyl fluoride and trifluoroethyl fluoride, which is applied in the field of continuous preparation of trifluoroacetyl fluoride, can solve the problems of high equipment cost, unfavorable industrial implementation, high cost of electrolytic plate production, low electrolytic efficiency and product purity, and achieves Avoid recycling and waste liquid pollution, easy large-scale industrial production, and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
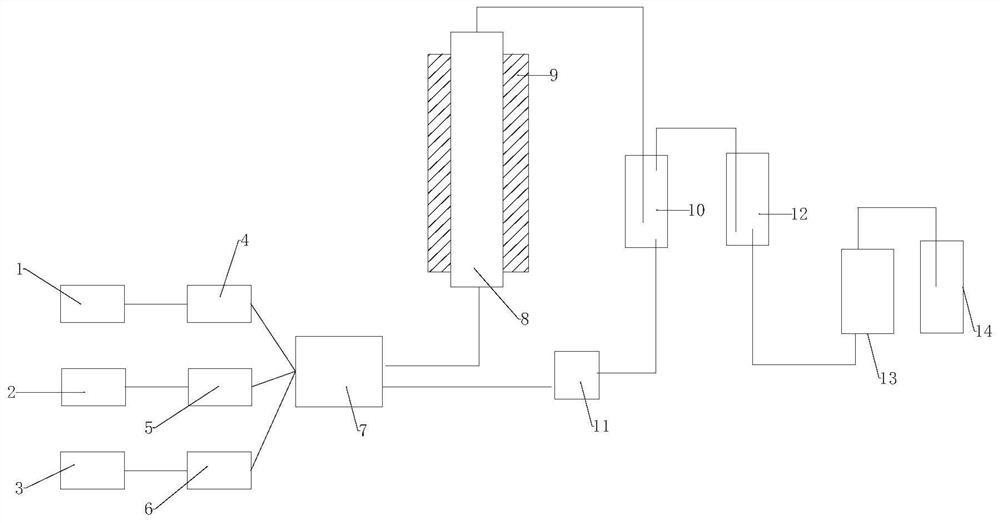
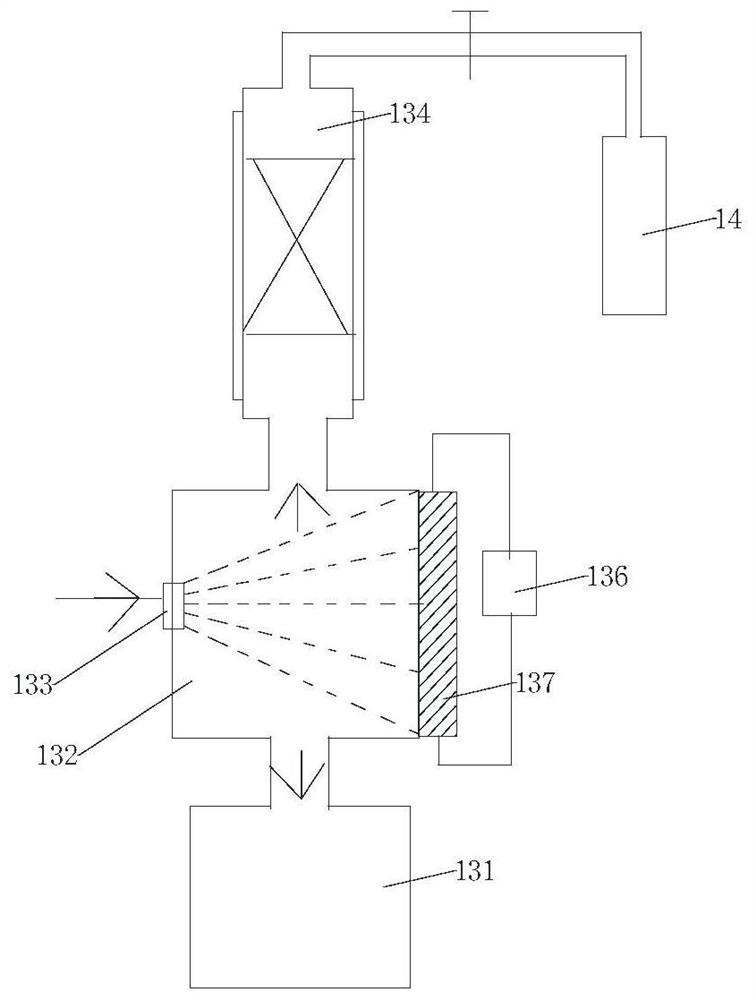
[0020] Example 1: Anhydrous hydrogen fluoride vaporization chamber temperature 25°C, trifluoroacetic acid vaporization chamber temperature 75°C, fluorinating reagent ClCHFCF 2 N(C 2 h 5 ) 2 The temperature of the gasification chamber is 40°C. After gasification of anhydrous hydrogen fluoride, trifluoroacetic acid, and fluorinated reagents, they enter the mixed gas transition chamber through the gas quality controller at the ratio of gas to substance: 1:1:1, and the resulting mixed gas is produced by Slowly pass through the catalyst packing column from bottom to top, the column temperature is 80°C; the packing column is equipped with a high-activity catalyst, wherein the high-activity catalyst is a supported catalyst that uses activated carbon as a carrier and contains catalyst active components; the catalyst active component For three (pentafluorophenyl) borane. The gas obtained from the outlet at the upper end of the catalyst packed column passes through the first cold tra...
Embodiment 2
[0024] Example 2: Anhydrous hydrogen fluoride vaporization chamber temperature 25°C, trifluoroacetic acid vaporization chamber temperature 75°C, fluorinating reagent ClCHFCF 2 N(C 2 h 5 ) 2 The temperature of the gasification chamber is 40°C. After gasification of anhydrous hydrogen fluoride, trifluoroacetic acid, and fluorinated reagents, they enter the mixed gas transition chamber through the gas quality controller at the ratio of gas to substance: 1:1:1, and the resulting mixed gas is produced by Slowly pass through the catalyst packing column from bottom to top, the column temperature is 80°C; the packing column is equipped with a high-activity catalyst, wherein the high-activity catalyst is a supported catalyst that uses activated carbon as a carrier and contains catalyst active components; the catalyst active component For three [3,5-bis (trifluoromethyl) phenyl] borane. The gas obtained from the outlet at the upper end of the catalyst packed column passes through the...
Embodiment 3
[0025] Example 3: Anhydrous hydrogen fluoride vaporization chamber temperature 25°C, trifluoroacetic acid vaporization chamber temperature 75°C, fluorinating reagent ClCHFCF 2 N(C 2 h 5 ) 2 The temperature of the gasification chamber is 40°C. After gasification of anhydrous hydrogen fluoride, trifluoroacetic acid, and fluorinated reagents, they enter the mixed gas transition chamber through the gas quality controller at the ratio of gas to substance: 1:1:1, and the resulting mixed gas is produced by Slowly pass through the catalyst packing column from bottom to top, the column temperature is 80°C; the packing column is equipped with a high-activity catalyst, wherein the high-activity catalyst is a supported catalyst that uses activated carbon as a carrier and contains catalyst active components; the catalyst active component for alumina. The gas obtained from the outlet at the upper end of the catalyst packed column passes through the first cold trap to remove trifluoroacet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com