Preparation method of O-monoacetyl ganciclovir
A technology of ganciclovir and monoacetyl, which is applied in the field of preparation of O-monoacetyl ganciclovir, can solve the problems of difficult removal of ganciclovir, difficulty in removing ganciclovir, and troublesome handling, and achieves short process steps. , The effect of reducing production cost and less impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] S1. Put 40kg of triacetylganciclovir and 120kg of pyridine into a 500L reaction kettle, add 4kg of water, stir and heat up to 70°C;
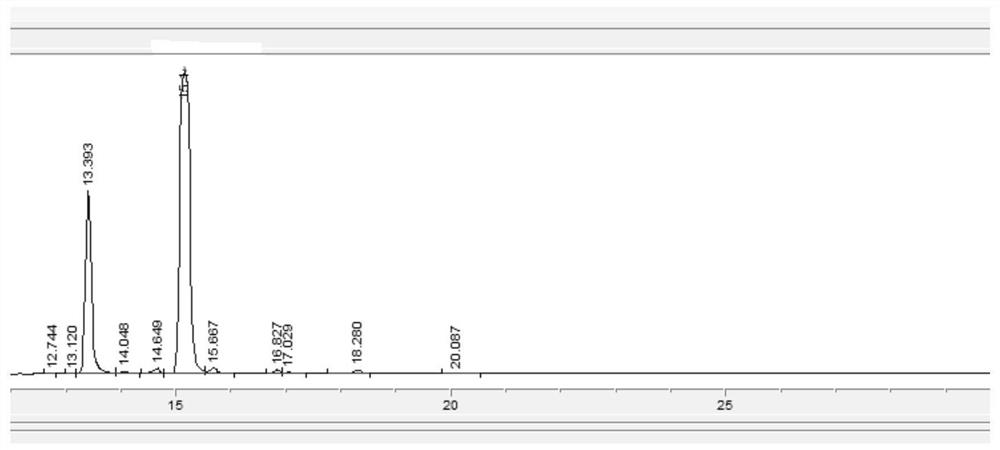
[0028] S2, sampling detection (HPLC) after 12 hours of incubation reaction, detection result is as follows figure 1 and Table 1, by figure 1 As can be seen from Table 1, the content of monoacetylganciclovir is obviously increasing, and the content of triacetylganciclovir is declining;
[0029] S3. After 36 hours of heat preservation reaction, sampling and testing were carried out, and the test results were as follows: figure 2 and Table 2, by figure 2 As can be seen from Table 2, the insulation reaction has reacted completely;
[0030] S4. Heating up to 100° C. and depressurizing, collecting evaporated water vapor and pyridine. At this time, the liquid main reactant and part of water are retained in the reactor, and then the pyridine is evaporated to dryness under reduced pressure for recycling;
[0031] S5, add 200kg ethyl acetate ...
Embodiment 2
[0039] S1. Put 40kg of triacetylganciclovir and 120kg of piperidine into a 500L reaction kettle, add 4kg of water, stir and heat up to 80°C;
[0040] S2, sampling and detection (HPLC) after 12 hours of heat preservation reaction, half of the reaction is completed;
[0041] S3. After 36 hours of heat preservation reaction, sampling and testing were performed, and the reaction was complete;
[0042] S4. Warming up to 90° C. and reducing pressure, collecting evaporated water vapor and piperidine. At this time, the liquid main reactant and part of water are retained in the reactor, and then the piperidine is evaporated to dryness under reduced pressure for recycling;
[0043] S5, add 200kg ethyl acetate to the reaction kettle, stir and reflux for 3 hours, cool down and crystallize;
[0044] S6, centrifugal treatment, after solid-liquid separation is realized, the solid is collected and dried to obtain 29.6 kg of O-monoacetylganciclovir finished product; the liquid is collected, d...
Embodiment 3
[0047] S1. Put 40kg of triacetylganciclovir and 120kg of N-methylmorpholine into a 500L reactor, add 4kg of water, stir and heat up to 75°C;
[0048] S2, sampling and detection (HPLC) after 12 hours of heat preservation reaction, the reaction has not been completed yet;
[0049] S3, take a sample after 40 hours of heat preservation reaction, and the reaction is complete;
[0050] S4. Heating up to 95°C and depressurizing, collecting the evaporated water vapor and N-methylmorpholine. At this time, the liquid main reactant and part of water are kept in the reaction kettle, and then the N-methylmorpholine is decompressed separately Evaporate to dryness and recycle;
[0051] S5, add 200kg ethyl acetate to the reaction kettle, stir and reflux for 3 hours, cool down and crystallize;
[0052] S6. Centrifuge and dry to obtain 29.5 kg of O-monoacetylganciclovir finished product, and distill to recover ethyl acetate.
[0053] It can be calculated that the yield of O-monoacetylgancicl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com