Universal quality control product preservation solution for in-vitro diagnostic kit
An in vitro diagnosis and kit technology, applied in the preservation of microorganisms and other directions, can solve the problems of complex preparation process and large limitations, and achieve the effect of simple preparation, taking into account safety and good compatibility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
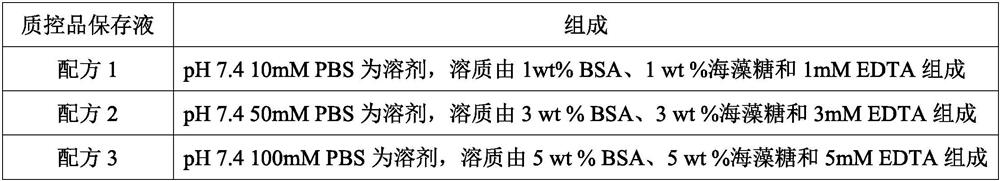
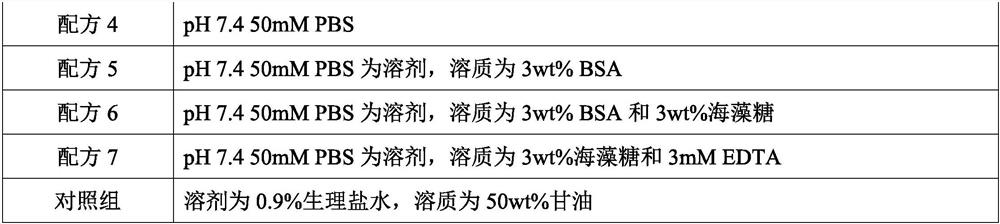
[0023] According to the formula in Table 1 below, use the conventional dissolution method to prepare the quality control product preservation solution:
[0024] Table 1
[0025]
[0026]
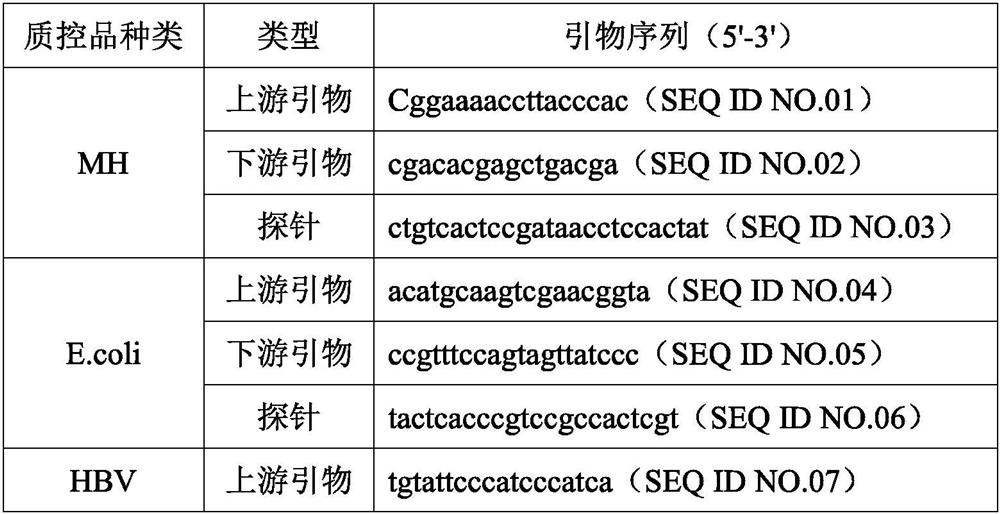
[0027] The quality control product that this embodiment is used for checking preservation effect is mycoplasma (MH) quality control product, escherichia coli (E.coli) quality control product, HBV pseudovirus quality control product (DNA class pseudovirus), COVID-19 pseudovirus Quality control (RNA-like pseudovirus). Among them, Mycoplasma hominis and Escherichia coli are natural inactivated bacteria, HBV pseudovirus and COVID-19 pseudovirus are artificially synthesized, and they all contain corresponding target gene fragments.
[0028] Preparation of MH quality control product: MH mycoplasma is purchased from outside, and diluted with the quality control product preservation solution to the corresponding concentration to obtain the MH quality control product.
[0029] Preparation of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com