Method for rapidly evaluating in-vitro antagonistic helicobacter pylori activity
A Helicobacter pylori, antagonistic technology, applied in the field of microbial detection, can solve the problems of large number of plates, flow away, small inhibition zone, etc., and achieve the effects of simplified operation steps, intuitive observation results, and practical detection methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
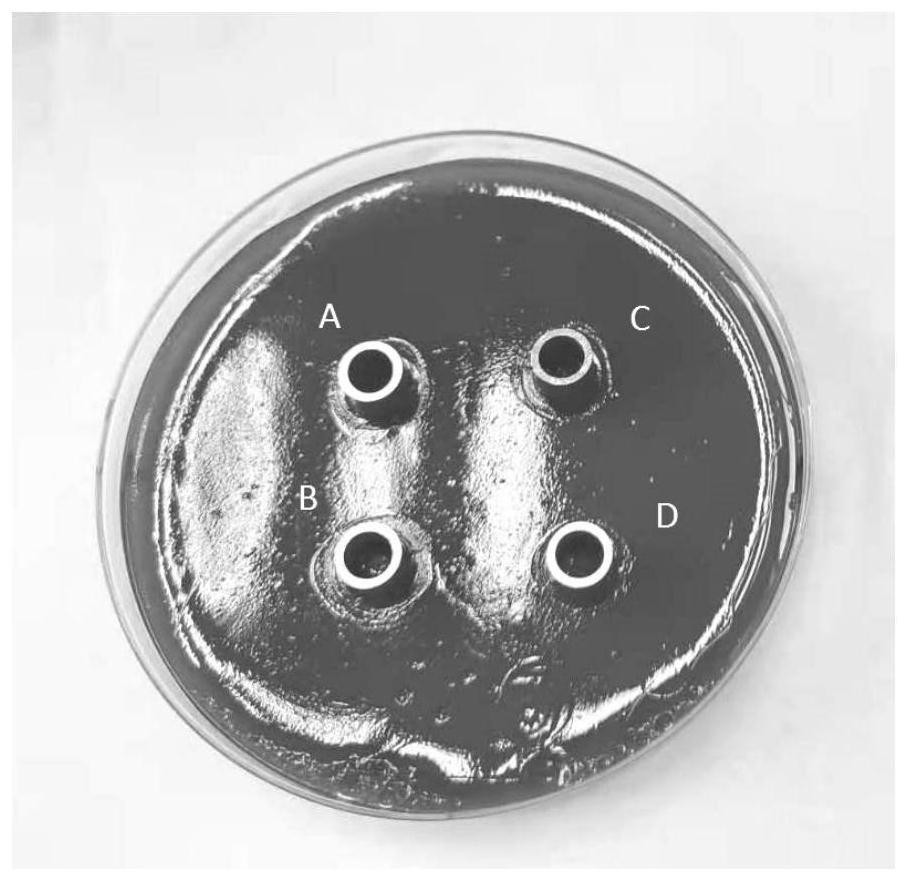
[0024] In the present invention, a sheep blood agar medium plate is taken, a bacterial suspension of Helicobacter pylori is coated on the sheep blood agar medium plate, an Oxford cup is placed, the sample solution to be tested is added into the Oxford cup, cultured for 3 to 5 days, and measured The diameter of the zone of inhibition. In the present invention, the culture conditions are preferably 37° C., 10% carbon dioxide, and 5% oxygen. In the present invention, the raw materials for the preparation of the sheep blood agar medium include the following components in parts by weight: 8-10 parts of brain heart extract powder, 20-25 parts of Columbia agar, 700-850 parts of water and defibrated sheep blood 30-40 servings. In the present invention, the preparation method of the sheep blood agar medium includes: mixing brain heart extract powder, Columbia agar and water, and adding defibrated sheep blood after autoclaving and cooling to 45-50°C. In the present invention, the thic...
Embodiment 1
[0030] 1. Cultivation and passage of Helicobacter pylori: Take the cultured sheep blood agar medium plate with the Helicobacter pylori ATCC43504 strain, add 800 μL of brain heart infusion broth (BHI) washing solution under sterile conditions, and the bacteria liquid Transfer to a 2mL sterile sample tube, take 100 μL of the bacterial solution onto the sheep blood agar medium, shake well, and incubate at 37°C with 10% carbon dioxide and 5% oxygen for later use.
[0031] The components of sheep blood agar medium are: brain heart extract powder 9.6g, Columbia agar 23.4g, distilled water 780mL, after autoclaving and cooling down to 46°C, add 33-40mL of defibrated sheep blood, pour plate.
[0032] The components of BHI lotion are: peptone 10.0g / L, dehydrated calf brain extract powder 12.5g / L, dehydrated beef heart extract powder 5.0g / L, sodium chloride 5.0g / L, glucose 2.0g / L, phosphoric acid Sodium hydrogen disodium 2.5g / L, water balance, pH 7.4.
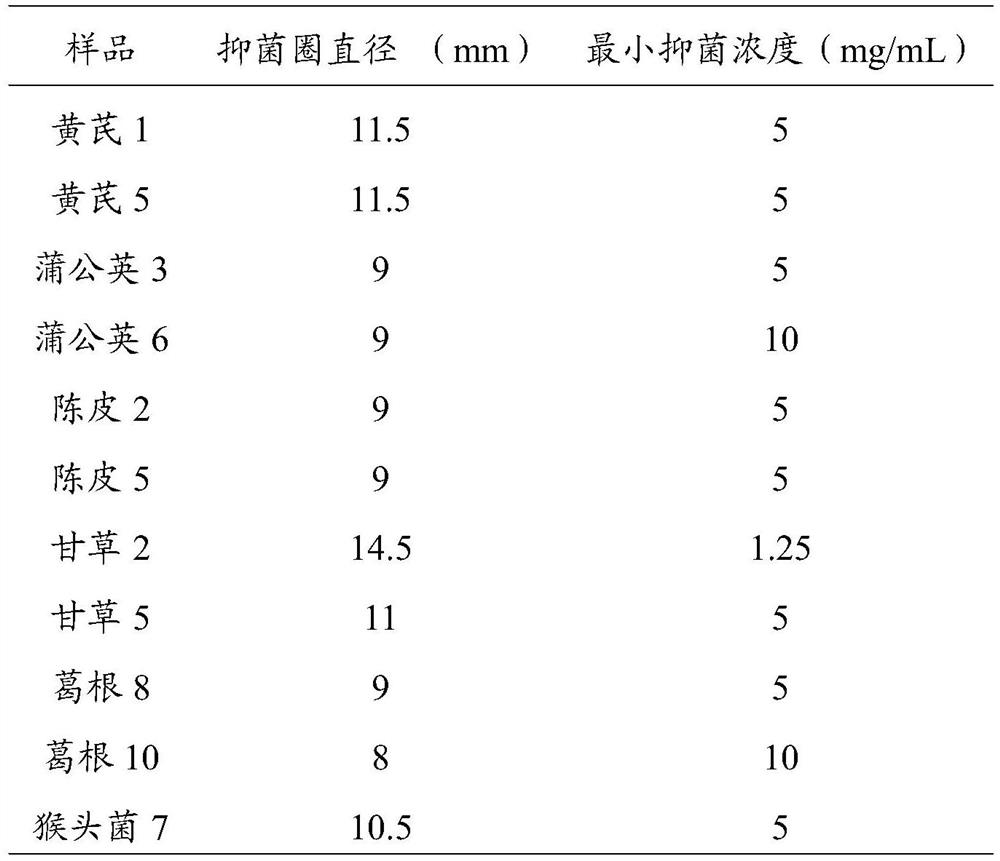
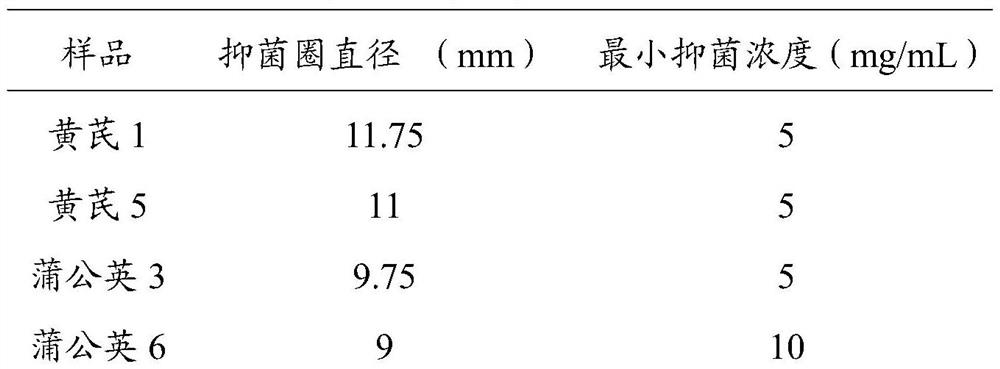
[0033] 2. Prepare the sample soluti...
Embodiment 2
[0040] 1. Cultivation and passage of Helicobacter pylori: Take the cultured sheep blood agar medium plate with Helicobacter pylori Sydney SS1 strain, add 800 μL of brain heart infusion broth (BHI) washing solution under sterile conditions, and the bacteria The solution was transferred to a 2mL sterile sample tube, and 100 μL of the bacterial solution was placed on the sheep blood agar medium, and after shaking, it was cultured at 37°C with 10% carbon dioxide and 5% oxygen for later use. The components of the sheep blood agar medium and the components of the BHI washing solution are the same as in Example 1.
[0041] 2. The configuration of the sample solution is the same as in Example 1.
[0042] 3. Determination of the diameter of the inhibition zone by the Oxford cup method: take a sheep blood agar medium plate with a diameter of 9 cm and a thickness of about 5 mm, and add about 100 μL to each plate to a concentration of 1×10 8 The bacterial suspension of Helicobacter pylor...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com