Enteric hard capsule
An enteric-coated, hard capsule technology, applied in the direction of capsule delivery, organic active ingredients, non-central analgesics, etc., can solve the problem that there is no special regulation on the temporary dissolution characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0354] In the preparation solution of the present invention and the method for preparing the preparation solution of the present invention, a step C of adding component iii can be added to step A or step B. Alkyl (meth)acrylate copolymers as component iii have little interaction with component i or component ii affecting viscosity or chemical stability and can thus be added after step A or step B as an aqueous dispersion .
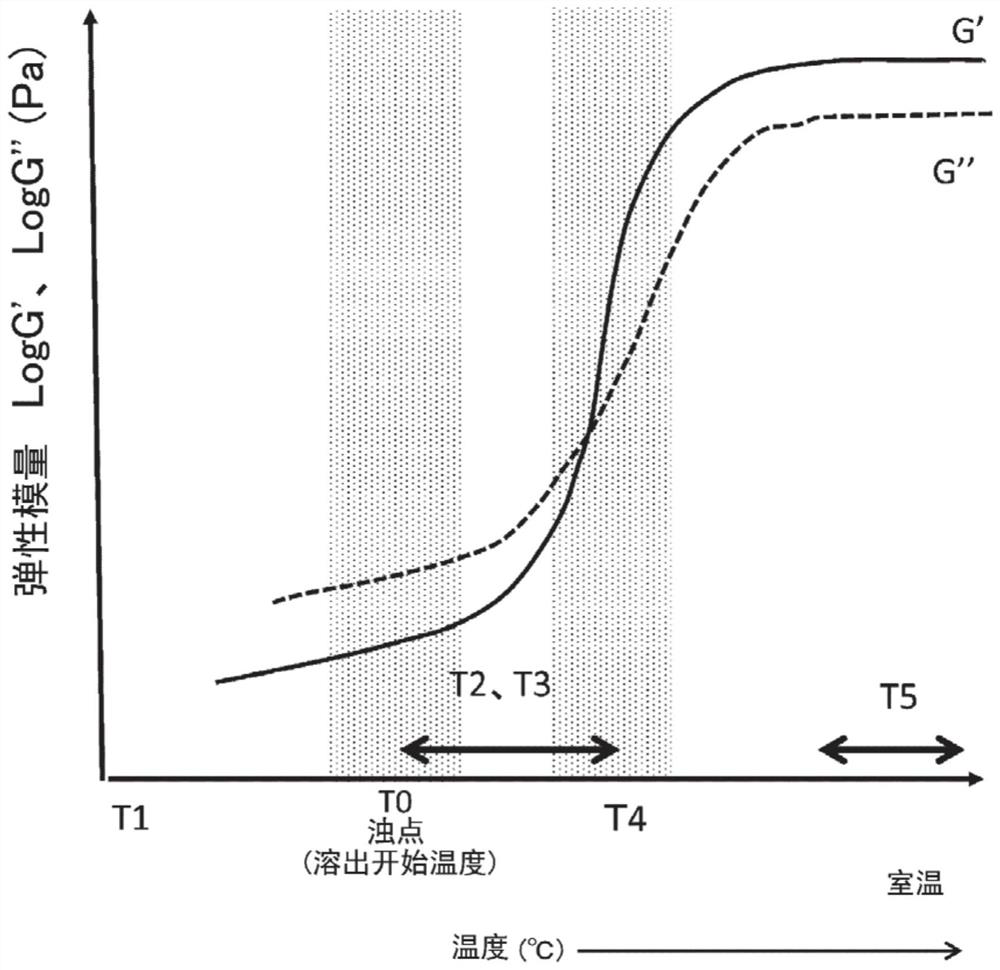
[0355] In addition, aspect 3-1 may further include: step D of maintaining the solution obtained in step B or C at a third temperature T3 lower than the cloud point of component i. In addition, the third temperature T3 is preferably higher than T2 and not lower than the cloud point T0 by more than 10°C. As a result, the partially dissolved state of component i can be stably maintained. For example, temperature T3 may be in the range of 30°C to 60°C. Preferably, the temperature T3 may be in the range of 50°C to 60°C.
[0356] Component iv of the preparat...
Embodiment
[0431] I. Materials used
[0432] Materials used in Examples, Reference Examples, and Comparative Examples are as follows.
[0433] 1. Water-soluble cellulose compound
[0434] As methylcellulose (MC) and hydroxypropylmethylcellulose (HPMC), METOLOSE (trademark) series, TC-5 (trademark) series, and SH series manufactured by Shin-Etsu Chemical Co., Ltd. were used. As hydroxypropylcellulose (HPC), NISSO HPC series from Nippon Soda Co., Ltd. was used. Specific product names and their substitution types and "viscosity values" (labeled viscosity or viscosity grades) are shown in Table 2.
[0435] The particle size distribution of TC-5 series and SH series HPMC was measured with a laser diffraction particle size distribution analyzer ("Microtrack particle size distribution diameter MT3300IIEX" manufactured by Microtrack BEL). The average particle size is in the range of 50-100 μm. The volume fraction of particles having a particle size of 100 μm or more is 50% or more.
[0436] [...
preparation example V-1
[0492] In the following examples and comparative examples, capsule preparation liquids were prepared according to Preparation Example III-1 (preparation method of aspect 3-1), and molded according to molding method IV. Based on their total mass (the total mass of the polymer components) expressed as 100% by mass, the mass percentages of the solid content of component i, component ii, component iii and component iv are expressed as α, β, γ, respectively and δ. Based on the total mass of the above-mentioned polymer components, the mass ratio of the basic neutralizer (NaOH) to other salts and the mass ratio of titanium oxide (opacifier) are expressed as ε (%) and σ (%), respectively. The mass ratio of the polymer components of components i to iv is defined as the polymer component concentration (%) based on the total mass of purified water as a solvent and the solid contents of components i to iv. Each specific composition is shown in Table 3. Furthermore, in these tables, th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com