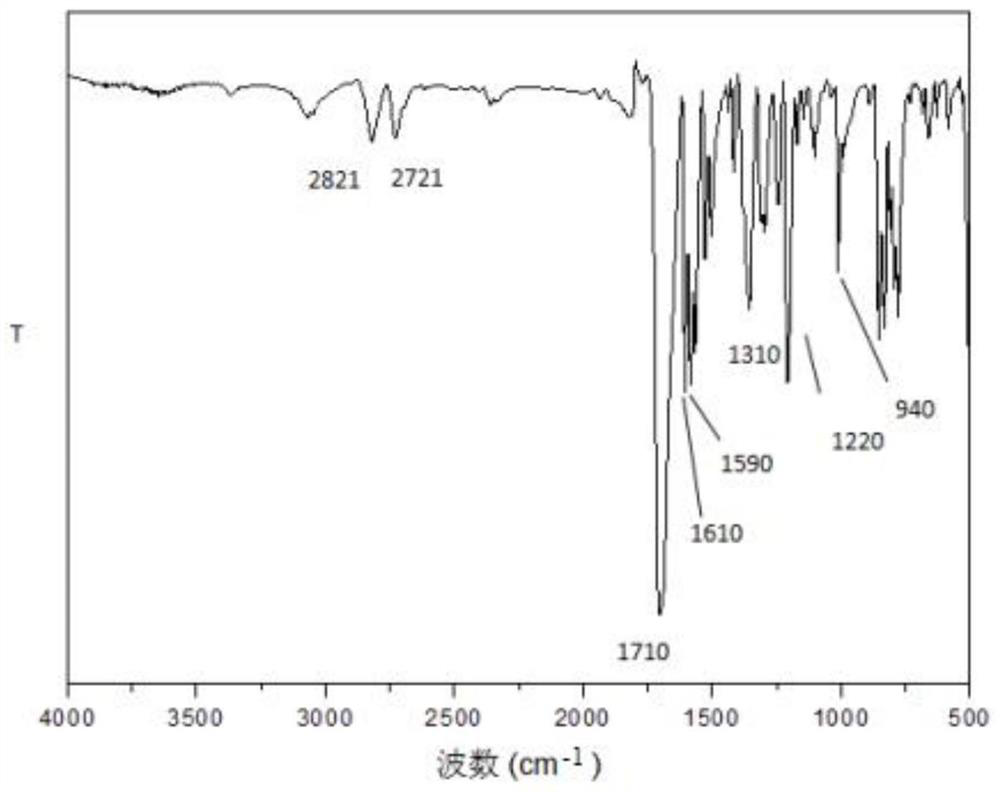
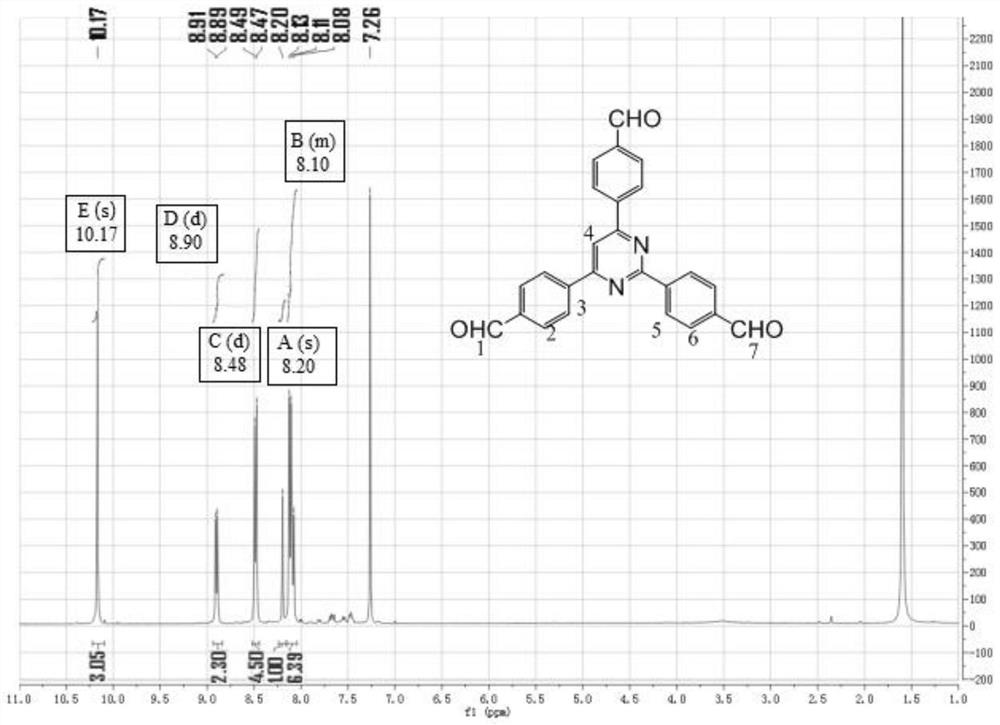
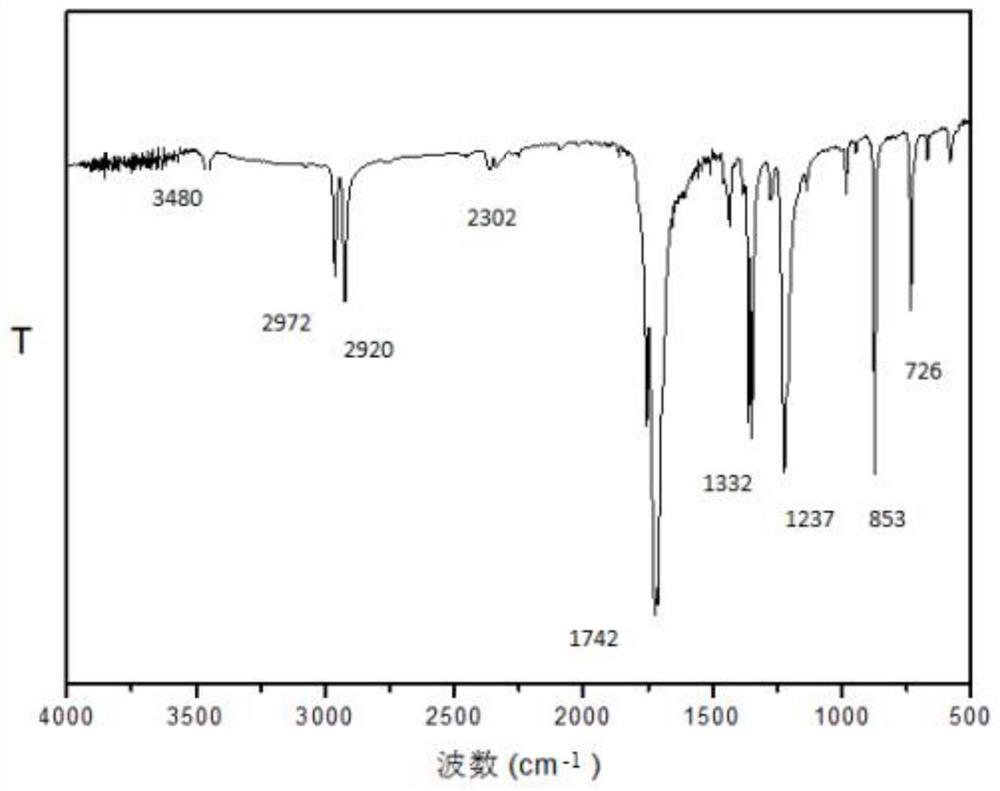
Conjugated microporous polymer based on 2,4,6-tri(4-formylphenyl)pyrimidine and preparation method of conjugated microporous polymer
A technology of formylphenyl and conjugated micropores, which is applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, chemical/physical processes, etc., can solve the needs of diverse CMPs, There are few types of CMPs, etc., to achieve the effect of large-scale production and simple and easy preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] The invention provides a method for preparing a conjugated microporous polymer based on 2,4,6-tris(4-formylphenyl)pyrimidine, comprising the following steps:
[0034] Mix 2,4,6-tris(4-formylphenyl)pyrimidine, symmetrical indacene-1,3,5,7(2H,6H)-tetraketone, organic solvent and acidic reagent, and prepare the resulting mixture The solution was subjected to aldol condensation reaction in a protective atmosphere to obtain a conjugated microporous polymer based on 2,4,6-tris(4-formylphenyl)pyrimidine.
[0035] The present invention has no special limitation on the source of the symmetric indacene-1,3,5,7(2H,6H)-tetraketone, which can be commercially available or self-made; in the present invention, the symmetric indacene Dashen-1,3,5,7(2H,6H)-tetraketone is preferably self-made, and the preparation method preferably includes the following steps:
[0036] Mix pyromellitic dianhydride, ethyl acetoacetate and triethylamine, heat in an oil bath to 60-65°C, add acetic anhydride...
Embodiment 1
[0067] Preparation of 2,4,6-tris(4-formylphenyl)pyrimidine comprises the following steps:
[0068] 2,4,6-trichloropyrimidine (0.3750g, 2mmol), 4-formylphenylboronic acid (1.4020g, 9mmol), potassium carbonate (0.7501g, 6mmol), cesium carbonate (1.9504g, 6mmol), four (Triphenylphosphine)palladium (3.5008g, 0.3mmol) was successively added into a round-bottomed flask and a magnet was placed, and then toluene (25mL), absolute ethanol (5mL) and distilled water (5mL) were successively added into the round-bottomed flask As a reaction solvent, place the round-bottomed flask in an oil bath, control the temperature of the oil bath to 100°C, and carry out the Suzuki coupling reaction under nitrogen protection and stirring for 72 hours at the beginning of system reflux; cool to room temperature after the reaction , add distilled water (100mL) and dichloromethane (100mL) to extract the product system obtained, separate to obtain the upper organic phase liquid, add anhydrous magnesium sulfa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com