IPMA neutralizing antibody detection method of CSFV
An antibody detection and cell technology, which is applied in the field of CSFV IPMA neutralizing antibody detection, can solve the problems that plague the pig industry, the diagnosis and prevention of swine fever, and achieve high sensitivity, simple operation, and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1CSFV virus isolation and identification
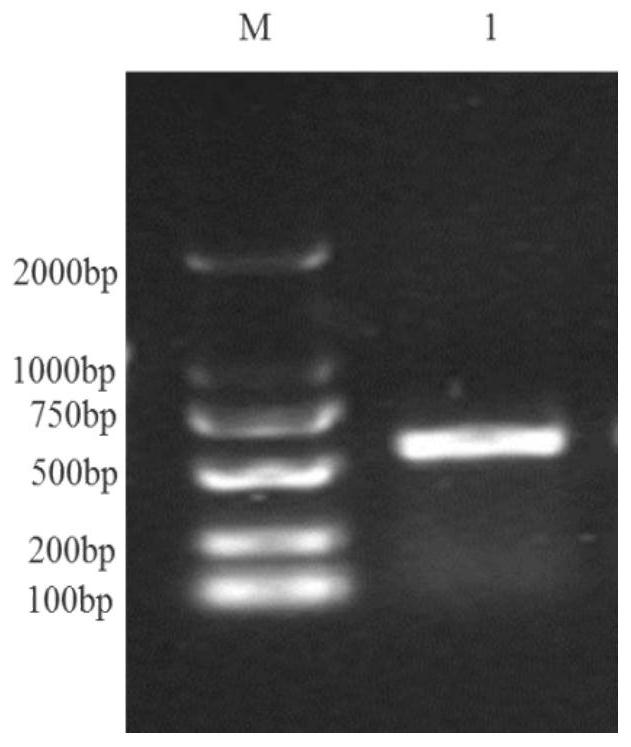
[0025] After washing the suspected CSFV disease tissue (lymph, liver, spleen, lung) with PBS for several times, add 1 mL of PBS to dissolve 1 mg of tissue sample, freeze and thaw repeatedly 3 times, take the supernatant, and then centrifuge at 12000 rpm for 5 min, and use 0.22 Filter with a μm filter to obtain CSFV virus liquid. Using a DNA extraction kit, extract the RNA of CSFV virus, and then reverse transcribe the RNA into cDNA. Use DNAMAN software to design primers for the target gene of CSFV, the amplified fragment is 531bp, and the specific primer sequences are as follows:
[0026] CSFV-F: 5'-cggctagcctgcaaggaagattac-3'; SEQ ID NO.1;
[0027] CSFV-R: 5'-tcatagatcttcattttccactgtggtgg-3'; SEQ ID NO.2.
[0028] Using the designed primers, PCR amplification was performed using the extracted cDNA as a template. The reaction system was as follows: template 1 μL, CSFV-F 0.5 μL, CSFV-R 0.5 μL, Ex Taq enzyme 12.5 μL...
Embodiment 2
[0030] Example 2 Establishment of Clinical Serum Neutralizing Antibody Detection Method
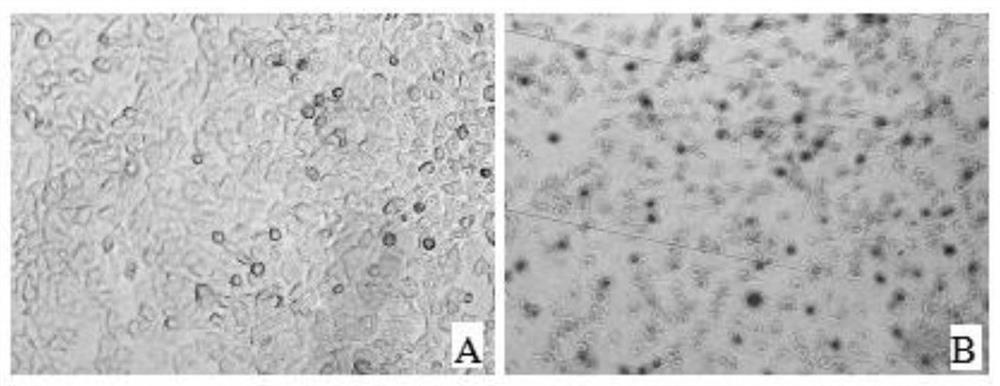
[0031] Digest and centrifuge PK15 cells to prepare 1.0×10 5 cells / mL cell suspension, spread 100 μL per well on a 96-well plate, and store at 37°C in 5% CO 2 Cultivate in the incubator for 12 hours. After the cells are completely adhered to the wall, incubate an equal volume of CSFV virus (50 μL) and clinical serum at 37°C for 1 hour, and then inoculate them on PK15 cells in a 96-well plate; at the same time, set up a control well for normal inoculated cells; After culturing for 48 hours, the 96-well plate was taken out after the cells were overgrown, and fixed with pre-cooled absolute ethanol. Add primary antibody: Wash the 96-well plate 2-3 times with PBS, then add 100 μL of CSFV-positive serum diluted 1:400 with 0.01M PBS (according to the detection method of IDEXX kit, CSFV-positive antibody obtained clinically), set Act at 37°C for 1h. Add secondary antibody: add 100 μL of goat an...
Embodiment 3I
[0033] Embodiment 3IPMA reaction conditions
[0034] 1) CSFV TCID 50 determination
[0035] (1) Spread the PK15 cell suspension on a 96-well plate, 100 μL per well, so that the cell volume reaches 2-3×10 5 cells / mL, cultivated for 12 hours until the cells were completely attached to the wall;
[0036] (2) In the penicillin bottle or the centrifuge tube, the CSFV virus liquid is diluted 10 times continuously, starting from 10 -1 -10 -10 ;
[0037](3) Inoculate the diluted virus onto a 96-well plate in which the cells grow into a single layer, inoculate a vertical row of 8 wells for each dilution, and inoculate 100 μL in each well;
[0038] (4) The remaining two vertical rows are not exposed to poison, and a normal cell control is set (100 μL of maintenance solution per well, and the maintenance solution is DMEM medium containing 2% fetal bovine serum);
[0039] (5) After culturing for 48 hours, the cells were taken out and fixed, and placed at -20°C for later use;
[004...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com