A kind of eutectic of azelaic acid and organic base and its preparation method and application
A technology of azelaic acid and organic base, which is applied in the field of eutectic and preparation of azelaic acid and organic base, which can solve the problems of instability and poor water solubility of azelaic acid, and achieve high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
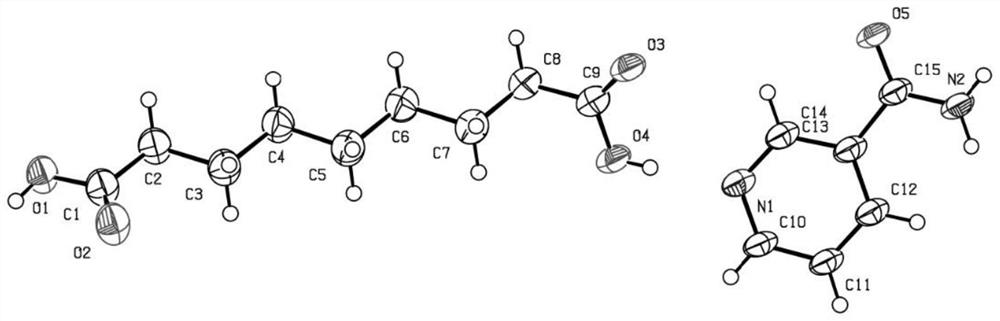
[0028] Such as Figure 5 Shown, the preparation method of the eutectic of a kind of azelaic acid of the present invention and organic base, comprises the following steps:
[0029] Step S100 , providing azelaic acid and an organic base, and mixing the azelaic acid and the organic base to obtain a mixture; wherein, the water solubility of the organic base is greater than that of the azelaic acid.
[0030] Specifically, azelaic acid may be 1,9-azelaic acid, and an organic base refers to an organic compound containing an amino group in its molecule. The organic base may be an alkaloid, and an alkaloid refers to a nitrogen-containing basic organic compound existing in nature. Specifically, the organic base may be one or more of niacinamide, matrine, choline, triethanolamine, acetyl-L-carnitine, cytisine and L-carnitine. The structural formula of 1,9-azelaic acid is as follows: The structural formula of niacinamide is as follows: The structural formula of L-carnitine is as fol...
specific Embodiment 1
[0050] A preferred method for the preparation of 3-pyridinecarboxamide 1,9-azelaic acid co-crystal:
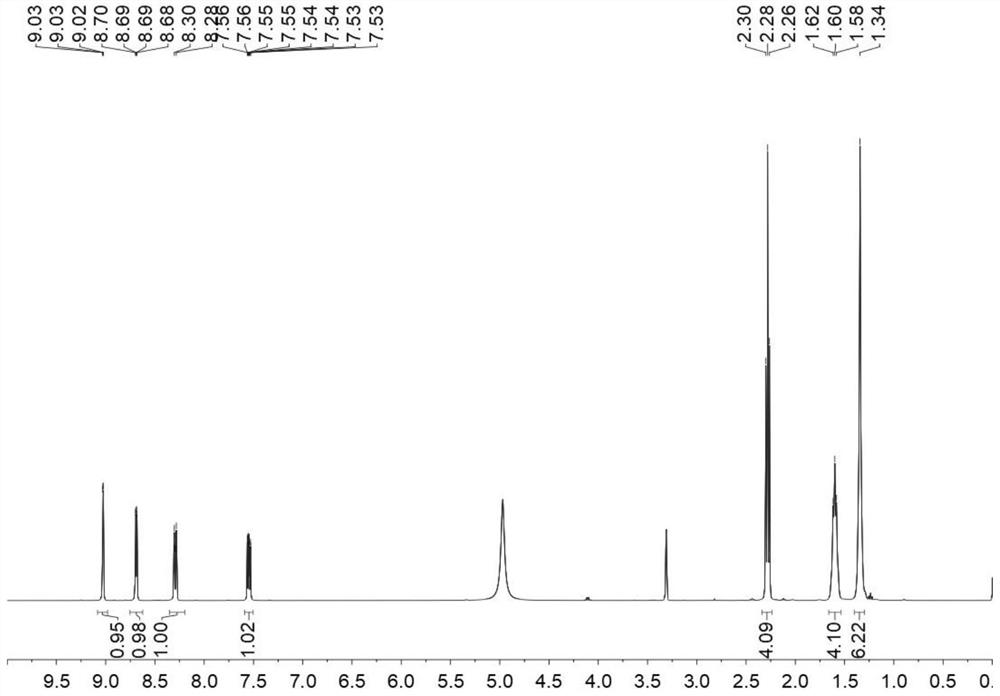
[0051] Step 1: Weigh 0.01 mol of 3-pyridinecarboxamide and 1,9-azelaic acid, place them in a reactor, and feed an inert gas into the reactor so that the entire reaction is carried out in an oxygen-free state. After sealing the reactor, the temperature was slowly raised to 115° C., and after reacting for 3 hours, the reactor was naturally cooled to room temperature to obtain the crude product of 3-pyridinecarboxamide 1,9-azelaic acid eutectic.
[0052] Second step: recrystallization of the crude eutectic product. Dissolve the crude product of 3-pyridinecarboxamide 1,9-azelaic acid eutectic with isopropanol:ethyl acetate at a ratio of 1:3 (according to the ratio of 6ml to 1g). After it is completely dissolved, filter the membrane to remove Impurities in the crude product. The eutectic solution after impurity removal was concentrated to a supersaturated state (the remaining vol...
specific Embodiment 2
[0093] A method for preparing 3-pyridinecarboxamide 1,9-azelaic acid co-crystal: Step 1: Place the reactor in an ice-salt bath at 0°C, weigh 0.01mol of 3-pyridinecarboxamide and add it to the reaction In the reactor, add 0.015mol of 1,9-azelaic acid into the reactor, and pass argon gas into the reactor, so that the whole reaction process is carried out in an oxygen-free state. After the reaction is sealed, the temperature is slowly raised to 85°C. After reacting for 6 hours, the eutectic solution of 3-pyridinecarboxamide 1,9-azelaic acid was obtained. Second step: recrystallization of the crude eutectic product. Dissolve the crude product of 3-pyridinecarboxamide 1,9-azelaic acid eutectic with isopropanol:ethyl acetate at a ratio of 1:3 (according to the ratio of 6ml to 1g). After it is completely dissolved, filter the membrane to remove Impurities in the crude product. The eutectic solution after impurity removal was concentrated to a supersaturated state (the remaining vol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
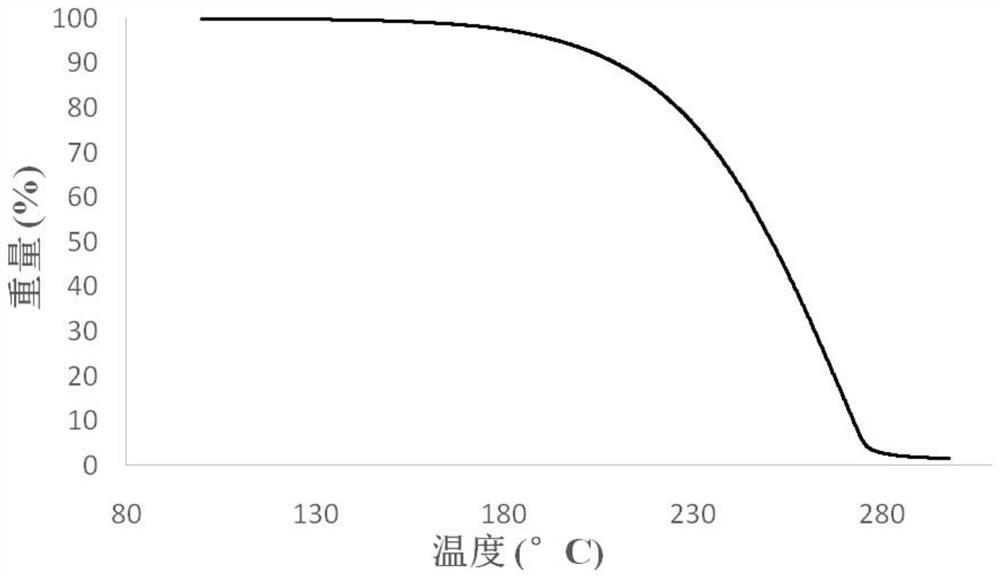
| decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com