Preparation method of biliverdin or derivatives thereof
The technology of a derivative and biliverdin is applied in the field of preparation of biliverdin or a derivative thereof, and can solve the problems of many reaction steps of dimethyl dipropionate, unsuitable for industrialized production, low yield of biliverdin, and the like, Achieve the effect of low cost, easy purification and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
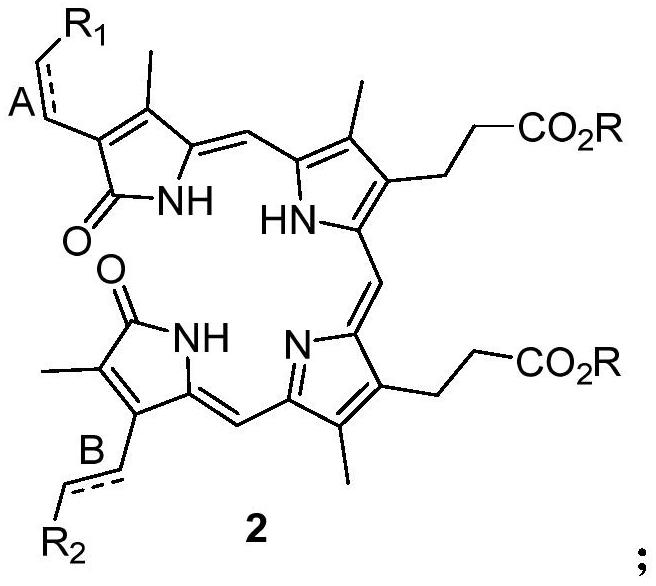
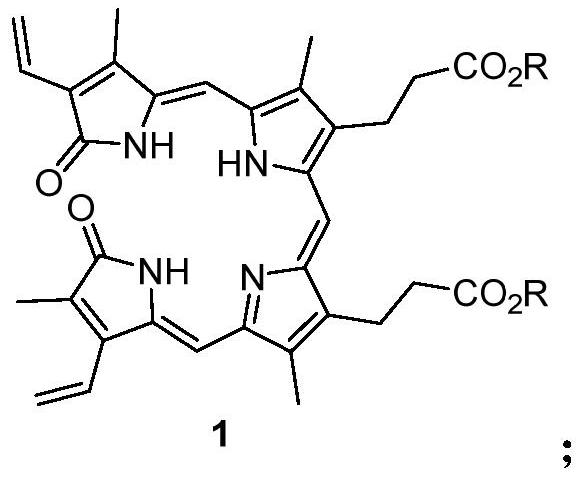
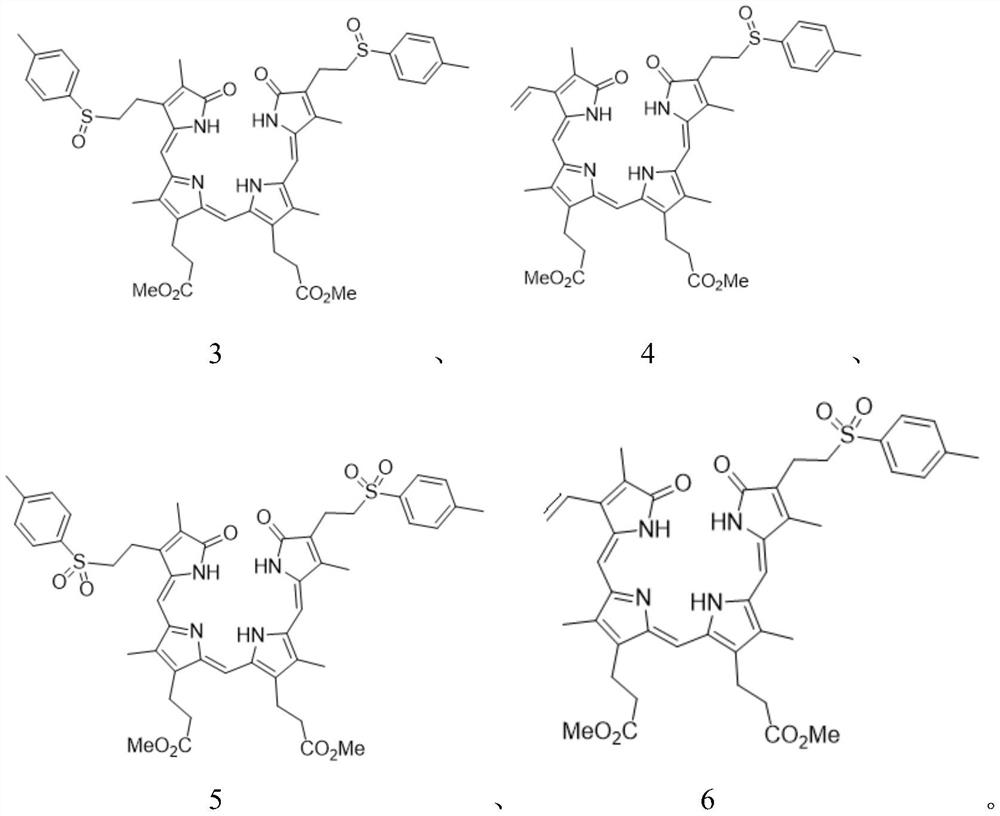
[0033] Weigh 0.92 grams of compound 3,3'-(3,18-bis(2-p-toluenesulfinylethyl)-2,7,13,17-tetramethyl-1,19-dioxo-1, 19,22,24-tetrahydro-21H-8,12-porphyrinyl)-dimethyl dipropionate (compound shown in formula 3), add 40 ml of xylene to dissolve, heat up to 135 ° C, keep stirring and react 2 Hours, the temperature was lowered, the solvent was evaporated under reduced pressure, and the residue was recrystallized from ethyl acetate to obtain 0.50 g of blue-green solid, which was biliverdin diester (compound shown in formula 7), and the yield was 63%. 1 H NMR (400MHz, CDCl 3 ): δ1.89(s, 3H), 2.10(s, 3H), 2.13(s, 3H), 2.20(s, 3H), 2.56(t, J=8.1Hz, 4H), 2.95(t, J= 8.1Hz, 4H), 3.69(s, 6H), 5.46(d, J=12.0Hz, 1H), 5.66(dd, J=12.0, 4.0Hz, 1H), 5.68(dd, J=16.0, 4.0Hz, 1H),6.02(s,1H),6.08(s,1H),6.14(dd,J=16.0,4.0Hz,1H),6.51(dd,J=16.0,12.0Hz,1H),6.64(dd,J =16.0,12.0Hz,1H),6.81(s,1H);ESI-Mass:633.20[M+Na] + .
[0034] The reaction formula is as follows:
[0035]
Embodiment 2
[0037]Weigh 0.92 grams of compound 3,3'-(3-vinyl-18-(2-p-toluenesulfinylethyl)-2,7,13,17-tetramethyl-1,19-dioxo- 1,19,22,24-tetrahydro-21H-8,12-porphyrinyl)-dimethyl dipropionate (compound shown in formula 4) as raw material, add 40 ml of DMF to dissolve, heat up to 130 ° C, keep warm The reaction was stirred for 2 hours, the temperature was lowered, the solvent was evaporated under reduced pressure, and the residue was recrystallized from ethyl acetate to obtain a blue-green solid, which was biliverdin diester (compound shown in formula 7), and the yield was 60%.
[0038] The reaction formula is as follows:
[0039]
Embodiment 3
[0041] Weigh 0.92 grams of compound 3,3'-(3,18-bis(2-p-toluenesulfinylethyl)-2,7,13,17-tetramethyl-1,19-dioxo-1, 19,22,24-tetrahydro-21H-8,12-porphyrinyl)-dimethyl dipropionate (compound shown in formula 3), add 40 ml of nitrobenzene to dissolve, heat up to 150°C, keep warm and stir for reaction After 2 hours, the temperature was lowered, the solvent was evaporated under reduced pressure, and the residue was recrystallized from ethyl acetate to obtain 0.50 g of blue-green solid, which was biliverdin diester (compound shown in formula 7), and the yield was 61%. 1 H NMR (400MHz, CDCl 3 ): δ1.89(s, 3H), 2.10(s, 3H), 2.13(s, 3H), 2.20(s, 3H), 2.56(t, J=8.1Hz, 4H), 2.95(t, J= 8.1Hz, 4H), 3.69(s, 6H), 5.46(d, J=12.0Hz, 1H), 5.66(dd, J=12.0, 4.0Hz, 1H), 5.68(dd, J=16.0, 4.0Hz, 1H),6.02(s,1H),6.08(s,1H),6.14(dd,J=16.0,4.0Hz,1H),6.51(dd,J=16.0,12.0Hz,1H),6.64(dd,J =16.0,12.0Hz,1H),6.81(s,1H);ESI-Mass:633.20[M+Na] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com