Teicoplanin preparation for injection and preparation method thereof
A technology of teicoplanin and injection preparation, applied in the field of medicine, can solve the problems of low yield, low purity, poor clarity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
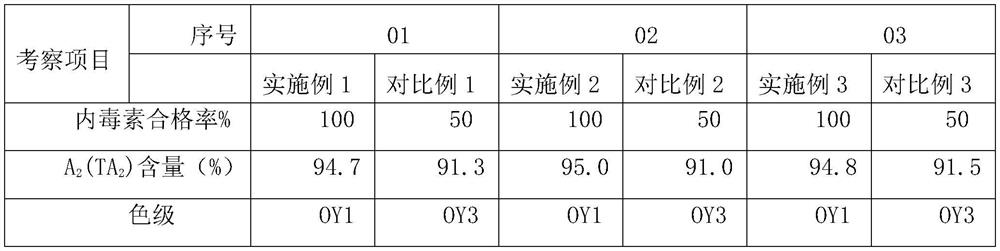
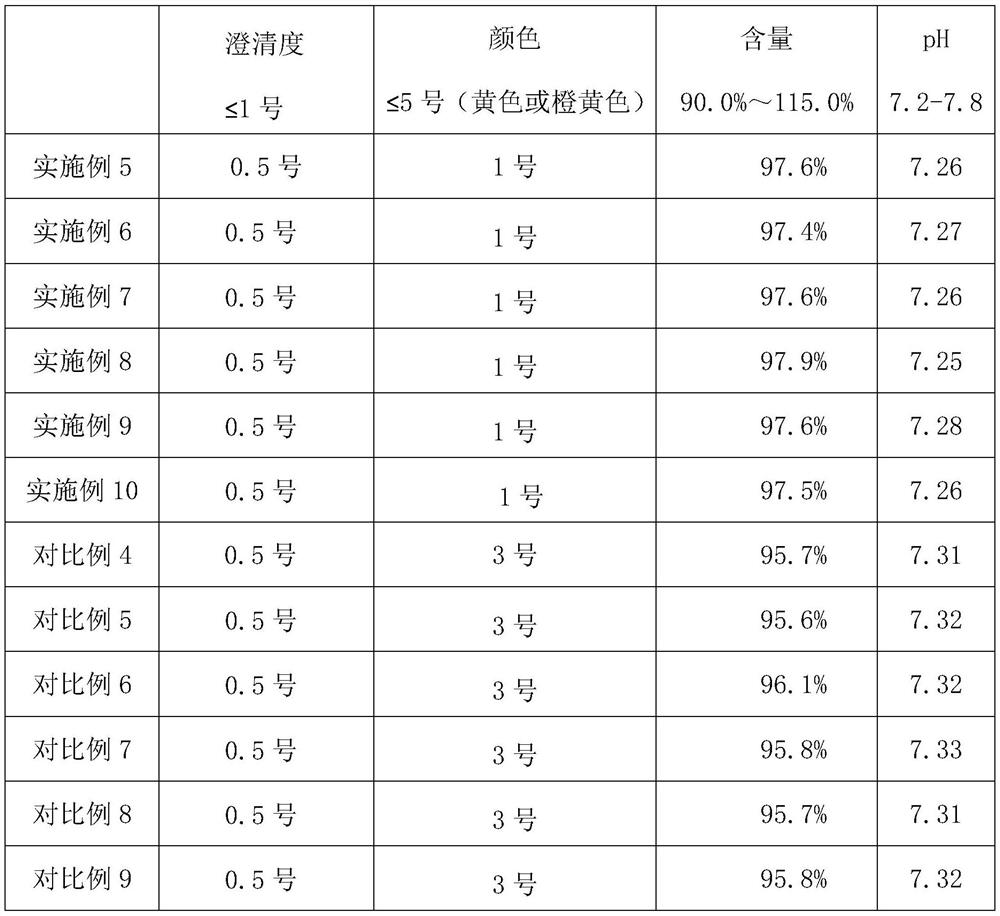
[0037] A. Adsorption: Teicoplanin fermentation filtrate totals 23.4 billion units, desorbs through macroporous adsorption resin to obtain a total of 16.97 billion units of teicoplanin raw material liquid;
[0038] B. Decolorization: Add 5.1 kilograms of 0.3% 767 injection activated carbon and 5.1 kilograms of 0.3% imported FSAC-01 activated carbon to the above-mentioned teicoplanin raw material solution for decolorization, and the total decolorization solution is 15.34 billion;
[0039] C. DAC column chromatography, the filler is polymer reversed-phase chromatography filler UniPs30-300, the teicoplanin filtrate is eluted through the column, and the total eluent collected is 8.37 billion;
[0040] D. Membrane system concentration: After the chromatographic solution is concentrated by ultrafiltration and then nanofiltration, the concentrated solution is washed with purified water to remove residual solvents, and a total of 8 billion teicoplanin concentrated solutions are obtained...
Embodiment 2
[0043] A. Adsorption: Teicoplanin fermentation filtrate has a total of 25.5 billion units, and a total of 20.87 billion units of desorption liquid is obtained by adsorption and desorption of macroporous adsorption resin;
[0044] B. Decolorization: Add 6.2 kg of 0.3% domestic activated carbon and 6.2 kg of 0.3% imported FSAC-01 activated carbon for decolorization, and the total decolorization solution is 15.9 billion;
[0045] C. DAC column chromatography, the filler is polymer reversed-phase chromatography filler UniPs30-300, and the decolorization solution is obtained by chromatography with a suitable chromatographic solution totaling 8.3 billion;
[0046]D. Membrane system concentration: After the chromatographic solution is concentrated by ultrafiltration and then nanofiltration, the molecular weight cut-off of the ultrafiltration membrane is 5000-10000 Daltons, and the molecular weight cut-off of the nanofiltration membrane is 200-300 Daltons. The concentrated solution is ...
Embodiment 3
[0049] A. Adsorption: Teicoplanin fermentation filtrate has a total of 26.6 billion units, and a total of 21.1 billion units of desorption liquid has been obtained by adsorption and desorption of macroporous adsorption resin;
[0050] B. Decolorization: Add 6.3 kg of 0.3% domestic activated carbon and 6.3 kg of 0.3% imported FSAC-01 activated carbon for decolorization, and the total decolorization solution is 15.77 billion;
[0051] C. Chromatography: the decolorized solution is obtained by chromatography with a suitable chromatographic solution totaling 7.9 billion;
[0052] D. Membrane system concentration: After the chromatographic solution is concentrated by ultrafiltration and then nanofiltration, the molecular weight cut-off of the ultrafiltration membrane is 5000-10000 Daltons, and the molecular weight cut-off of the nanofiltration membrane is 200-300 Daltons. The concentrated solution is washed with purified water Residual solvent was removed to obtain a total of 7.4 b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com