Refining method of favipiravir
A favipiravir and refining method technology, applied in new refining fields, can solve the problems of multiple impurities, low yield of body pure recrystallization, difficult to prepare high-purity favipiravir with high yield, and achieve simple Operation, the effect of excellent crystallinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
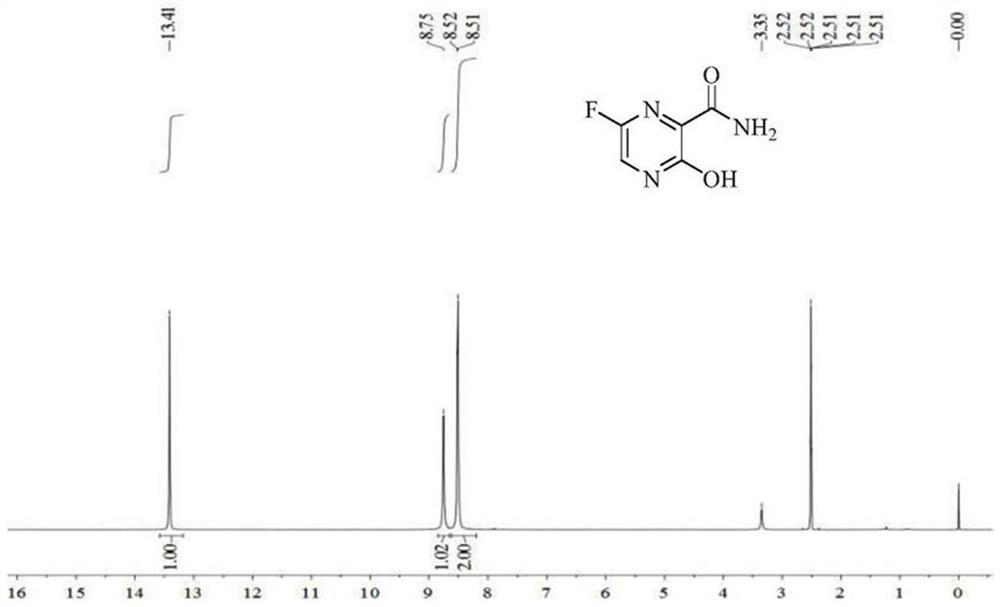
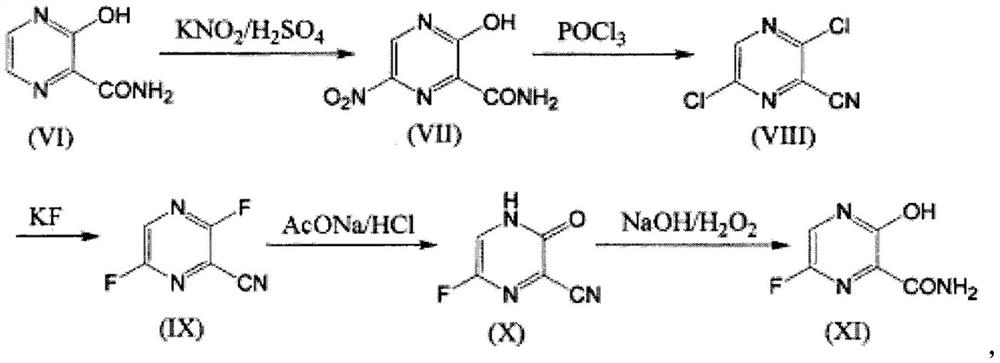
[0026] Embodiment 1: Preparation of 6-fluoro-3-hydroxyl-2-cyanopyrazine
[0027] Add 250mL of N,N-dimethylformamide and 52g of potassium fluoride into a 1L reaction flask, add 3,6-dichloro-2-cyanopyrazine (50g) into the reaction kettle, and then raise the temperature to 105- 112°C, heat preservation and stirring for 4-5 hours. After the heat preservation is over, the cold water is cooled to 30-40°C, and then the ice salt is continued to cool down to 0-15°C. Control the temperature at 0-15°C, add 30.3g of glacial acetic acid to the reaction bottle, after the addition is complete, continue to add 52g of triethylamine, and keep stirring at 0-15°C for 1 hour.
[0028] After the heat preservation is over, add 250g of water, stir for 20 minutes, add 85g of liquid caustic soda, adjust the pH to about 9.0, add 250ml of toluene, and extract once. Adjust the pH of the water phase to about 2 with concentrated hydrochloric acid, consume 12g of concentrated hydrochloric acid, add 300ml o...
Embodiment 2
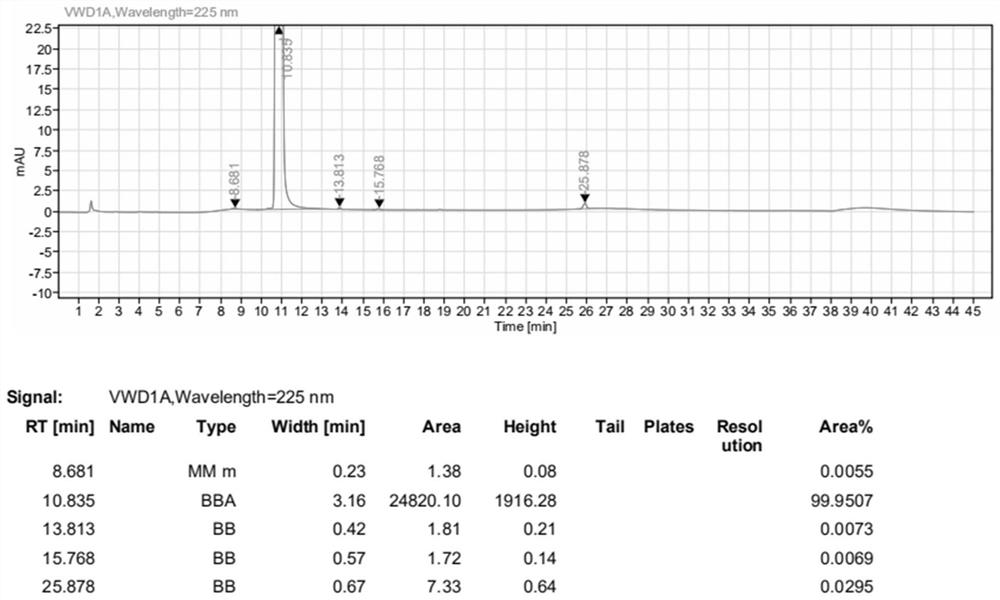
[0030] Add 67.2g30% liquid caustic soda to 350 milliliters of water, then add 35 grams of 6-fluoro-3-hydroxyl-2-cyanopyrazine prepared in Example 1, control the temperature at 10-20°C, add dropwise 30% hydrogen peroxide (85.6 g), reacted for 1 h, and TLC detected that the reaction of the raw materials was complete. Adjust the pH to 2.5-3 with hydrochloric acid, filter, rinse and dry. Dissolve the dried 28g of favipiravir in 400ml of ethyl acetate, raise the temperature to 70°C, add 24.4g of triethylamine dropwise, keep stirring for 1h, cool down to 0-5°C to crystallize for 1h, filter, and dry to obtain 63.5g. The dried Favipiravir (see the Raman spectrum figure 1 ) was dissolved in 150ml of water, adjusted to pH 2.5-3 with hydrochloric acid, filtered, rinsed, and dried to obtain 26.6g, with a molar yield of 95.5% and a purity of 99.9%.
Embodiment 3
[0032] Add 67.2g30% liquid caustic soda to 350 milliliters of water, then add 35 grams of 6-fluoro-3-hydroxyl-2-cyanopyrazine prepared in Example 1, control the temperature at 10-20°C, add dropwise 30% hydrogen peroxide (85.6 g), reacted for 1 h, and TLC detected that the reaction of the raw materials was complete. Adjust the pH to 2.5-3 with hydrochloric acid, filter, rinse and dry. Dissolve the dried 28g of Favipiravir in 400ml of ethyl acetate, raise the temperature to 80°C, add 35.7g of N,N-diisopropylethylamine dropwise, keep stirring for 1h, cool down to 0-5°C to crystallize for 1h, Filter and dry to obtain 68.4g. The dried Favipiravir (see the Raman spectrum figure 1 ) was dissolved in 150ml of water, adjusted to pH 2.5-3 with hydrochloric acid, filtered, rinsed, and dried to obtain 26.9g, with a molar yield of 96.1% and a purity of 99.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com