Method for purifying ferric trichloride
A technology of ferric chloride and a purification method, applied in directions such as the preparation of ferric halide and iron compounds, can solve the problems of limiting the application field of ferric chloride, unfavorable purification, single extraction characteristics of iron ions, etc., and achieves cost reduction and maintenance. Purity, volatile effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] A method for purifying ferric chloride, comprising the following steps:
[0030] S1. 1kg ferrous chloride solution A 0 Chlorine gas was introduced to make the Fe in the solution 2+ Oxidation is complete to obtain ferric chloride solution;
[0031] S2. Add 3kg of industrial hydrochloric acid and 2kg of butyl acetate to the ferric chloride solution, vibrate, let stand until the layers are complete, and take the organic layer;
[0032] S3. Add 1 kg of pure water to the organic layer for back extraction to obtain ferric chloride with ultra-low impurity content, and return the organic layer to the system for recycling;
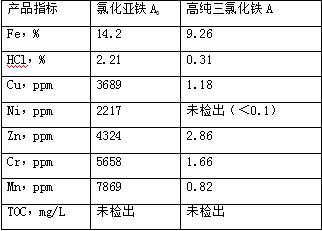
[0033] S4. Evaporate ferric chloride to boiling for 10 minutes to obtain high-purity ferric chloride A.
[0034] The experimental data are shown in the table below.
[0035]
Embodiment 2
[0037] A method for purifying ferric chloride, comprising the following steps:
[0038] S1. 2kg ferrous chloride solution A 0 Chlorine gas was introduced to make the Fe in the solution 2+ Oxidation is complete to obtain ferric chloride solution;
[0039] S2. Add 1kg of ferric chloride solution to 3kg of industrial hydrochloric acid and 2kg of butyl acetate, vibrate, let stand until the layers are complete, and take the organic layer;
[0040] S3 Add 1 kg of ferric chloride and 3 kg of industrial hydrochloric acid to the organic layer obtained in S2, oscillate and stand until the layers are complete, and take the organic layer;
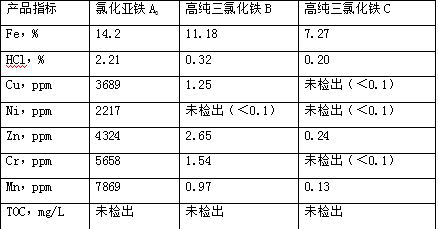
[0041] Add 1kg pure water in S4 organic layer and carry out stripping, obtain the iron trichloride B of low impurity content 1 , adding 1kg of pure water to the organic layer for stripping, to obtain iron trichloride C with ultra-low impurity content 1 , the organic layer is returned to the extraction system for recycling;
[0042] S4. Ferric tric...
Embodiment 3
[0046]A method for purifying ferric chloride, comprising the following steps:
[0047] S1. 1kg ferrous chloride solution A 0 Add appropriate amount of sodium chlorate and hydrochloric acid to make Fe in the solution 2+ Oxidation is complete to obtain ferric chloride solution;
[0048] S2. Add 2kg of industrial hydrochloric acid and 1kg of butyl acetate to the ferric chloride solution, vibrate and let stand until the layers are complete, then take the organic layer;
[0049] S3. Add 1 kg of pure water to the organic layer for back extraction to obtain ferric chloride with ultra-low impurity content, and return the organic layer to the system for recycling;
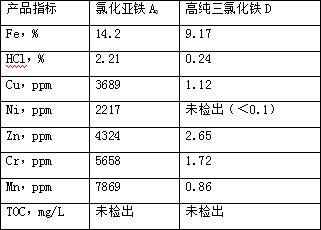
[0050] S4. Evaporate ferric chloride to boiling for 15 minutes to obtain high-purity ferric chloride D.
[0051] The experimental data are shown in the table below.
[0052]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com