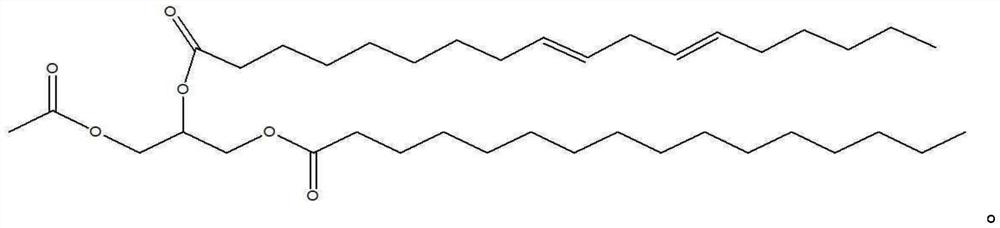
Method for producing 1-palmitoyl-2-linoleoyl-3-acetyl glycerol
A technology of palmitoylglycerol and acetylglycerol, which is applied in the preparation of carboxylic acid esters, asymmetric anhydrides, and carboxylic acid halides, etc., can solve the problems of reduced stability, reduced reaction yield, and decomposition of reaction products, and achieves inhibition Formation of impurities or degradation products, effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
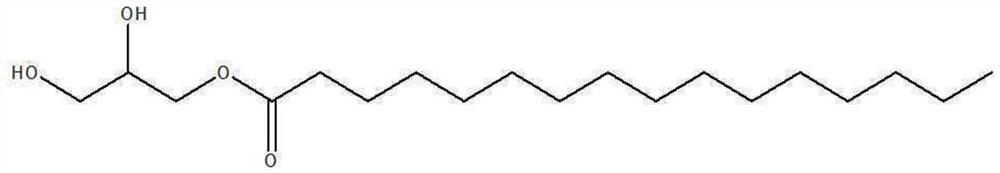
[0034] [Example 1] Preparation of 1-palmitoyl-3-acetylglycerol intermediate
[0035] Into a 250 mL three-neck round bottom flask were added 20 g of 1-palmitoylglycerol, 140 mL of dichloromethane, 33.5 g of pyridine, and 0.15 g of dimethylaminopyridine, and the mixture was heated to 30° C. to dissolve 1-palmitoyl glycerin. 3.1 g of acetic anhydride was added dropwise while maintaining the temperature of the reactant at 18°C, 5.7 g of acetyl chloride and 11 mL of dichloromethane were added dropwise while maintaining the temperature at 5°C, and the reaction was allowed to proceed for 18 hours. After the reaction was completed, 90 mL of purified water and 40 g of concentrated hydrochloric acid were added, the temperature was raised to 25° C., and the aqueous layer was separated to obtain an organic layer. The obtained organic layer was further subjected to a layer separation process using purified water again. Magnesium sulfate and potassium carbonate were added to the separate...
Embodiment 2
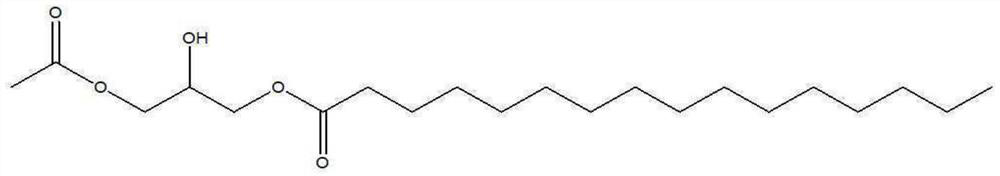
[0036] [Example 2] Preparation of 1-palmitoyl-3-acetylglycerol intermediate
[0037] 30 g of 1-palmitoylglycerol, 180 mL of dichloromethane, 50.3 g of pyridine, and 0.22 g of dimethylaminopyridine were added to a 250 mL three-neck round bottom flask, and the mixture was heated to 25-30° C. to dissolve 1- Palmitoyl Glycerin. While maintaining the temperature of the reactant at 18°C, 9.2 g of acetic anhydride was added dropwise. After the dropwise addition, the temperature was maintained at 5°C, and the reaction was performed for 22 hours.
[0038] After the reaction was completed, 90 mL of purified water and 40 g of concentrated hydrochloric acid were added, the temperature was raised to 25° C., and the aqueous layer was separated to obtain an organic layer. The obtained organic layer was further subjected to a layer separation process using purified water again. Magnesium sulfate and potassium carbonate were added to the separated organic layer, stirred for 1 hour, then ma...
Embodiment 3
[0039] [Example 3] Preparation of 1-palmitoyl-2-linoleoyl-3-acetylglycerol
[0040] Add 7.67g of linoleic acid, 80mL of n-hexane and 3.31g of pivaloyl chloride to a 250mL three-neck round bottom flask, and cool the reactant to -5~10°C, and dropwise add 5.97g of triethylamine . After adding 10 g of 1-palmitoyl-3-acetylglycerol and 0.328 g of 4-dimethylaminopyridine to the reactant, the reactant was reacted for 12 hours while stirring under nitrogen.
[0041] After the reaction was completed, 40 mL of purified water was added to the reactant, and the aqueous layer was subjected to layer separation to obtain a washed organic layer. Add 27 mL of methanol and 13.5 mL of a mixed solution of 0.1 N KOH to the separated organic layer, wash twice, then wash with 40 mL of a mixed solution of 95 volume % methanol and pure water, and wash with 10 mL of 0.1 volume % hydrochloric acid, It was then washed with 40 mL of 0.05% by weight aqueous sodium bicarbonate. To the washed organic lay...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com