A dual-response drug-releasing mofs@ibu nanofiber transdermal sustained-release material and its preparation method and application
A nanofiber and sustained-release material technology, applied in the field of biomedical materials, can solve problems such as severe gastrointestinal and side effects, and achieve the effects of single product, less side reactions, and mild preparation conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] A preparation method of MOFs@IBU nanofiber transdermal sustained-release material with dual-response drug release, comprising the steps of:
[0042] (1) Weigh 0.0505g of ligand 4,4'-((4-(4H-1,2,4-triazol-4-yl)benzene)azodioyl)dibenzoic acid, 0.074g of hexahydrate Zinc nitrate and 0.0505g of ibuprofen API were dissolved in 30ml of mixed solvent of pure water and N,N-dimethylformamide (DMF) (pure water: N,N-dimethylformamide (DMF) = 1:1 (v / v)), add 150uL of diethanolamine (DEA), and mix well. After standing at room temperature for 24 h, centrifugal filtration, washing with DMF three times, and vacuum drying at 63 °C to obtain a brown powder solid, namely MOFs@IBU solid. The microscopic morphology of the obtained MOFs@IBU is a flower-shaped structure with a particle size of 90-400 nm.
[0043] (2) 0.7 g of polyacrylonitrile (PAN, weight average molecular weight Mw=8 5000) was weighed and dissolved in 10 ml of N,N-dimethylformamide (DMF) to prepare a solution. 0.028 g of...
Embodiment 2
[0046] A preparation method of MOFs@IBU nanofiber transdermal sustained-release material with dual-response drug release, comprising the steps of:
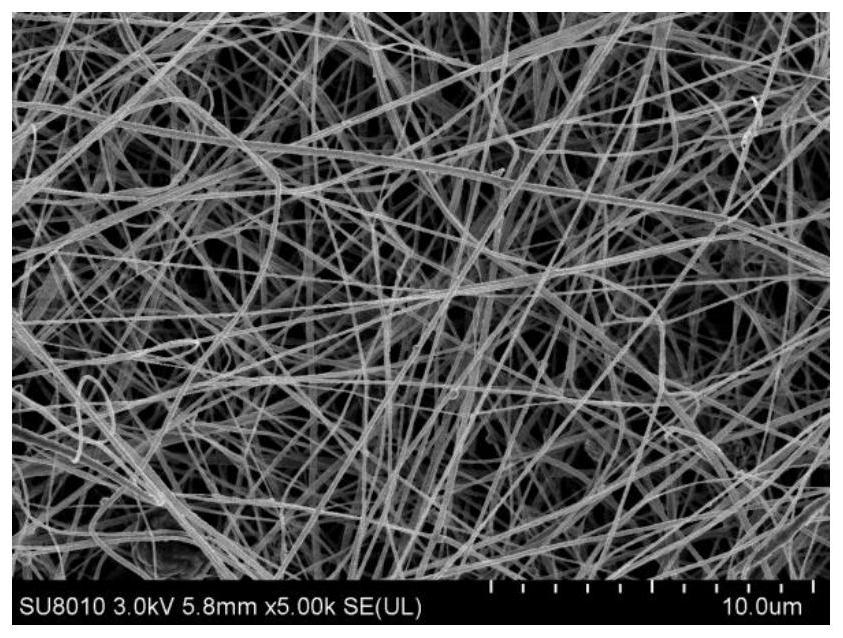
[0047] (1) Weigh out 0.0505g ligand 4,4'-((4-(4H-1,2,4-triazol-4-yl)benzene)azodioyl)dibenzoic acid and 0.0555g hexahydrate Zinc nitrate and 0.0505g of ibuprofen API were dissolved in 30ml of mixed solvent of pure water and N,N-dimethylformamide (DMF) (pure water: N,N-dimethylformamide (DMF) = 1:1 (v / v)), and add diethanolamine (DEA) 140uL, mix well. After standing at room temperature for 24 h, centrifugal filtration, washing with DMF three times, and vacuum drying at 68 °C to obtain a brown powder solid, namely MOFs@IBU solid. figure 1 For the SEM images of the resulting MOFs@IBU, from figure 1 It can be seen that the as-prepared MOFs@IBU have a flower-shaped structure with a size of about 90–300 nm.
[0048] (2) 0.7 g of polyacrylonitrile (PAN, weight-average molecular weight Mw=150000) was weighed and dissolved in 10 ml of N...
Embodiment 3
[0051] A preparation method of MOFs@IBU nanofiber transdermal sustained-release material with dual-response drug release, comprising the steps of:
[0052] (1) Weigh out 0.0505g ligand 4,4'-((4-(4H-1,2,4-triazol-4-yl)benzene)azodioyl)dibenzoic acid and 0.074g hexahydrate Zinc nitrate and 0.101g of ibuprofen API were dissolved in 30ml of mixed solvent of pure water and N,N-dimethylformamide (DMF) (pure water: N,N-dimethylformamide (DMF) = 1:1 (v / v)), add 150uL of diethanolamine (DEA), and mix well. After standing at room temperature for 24 h, centrifugal filtration, washing with DMF three times, and vacuum drying at 70 °C to obtain a brown powder solid, namely MOFs@IBU solid. figure 2 For the SEM images of the resulting MOFs@IBU, from figure 2 It can be seen that the as-prepared MOFs@IBU have a flower-shaped structure with a size of about 90–300 nm.
[0053] (2) 0.7 g of polyacrylonitrile (PAN, weight-average molecular weight Mw=150000) was weighed and dissolved in 10 ml o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com