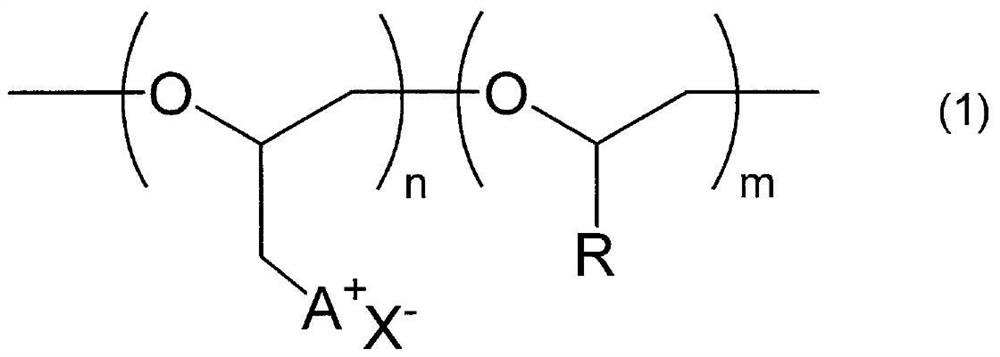
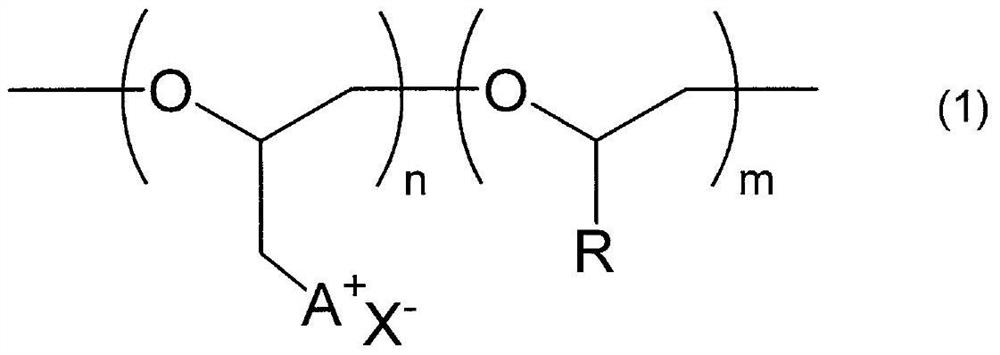
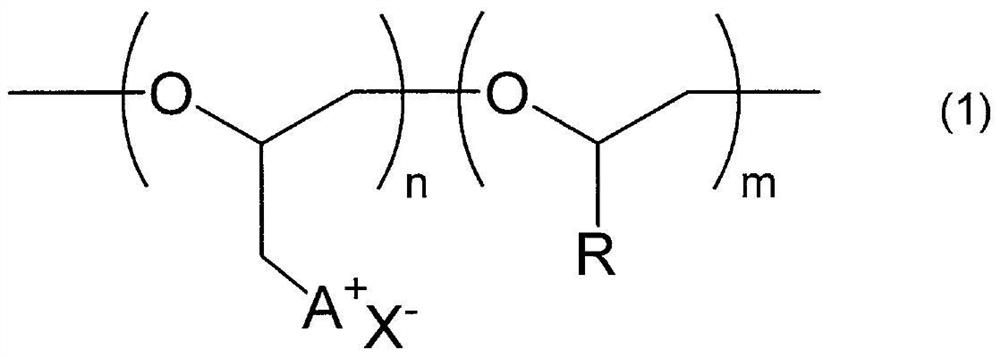
Polyether compound and gas separation membrane
A technology of polyether compounds and proprietors, which is applied in the field of polyether compounds and gas separation membranes, and can solve problems such as poor shape stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0167] Hereinafter, the present invention will be specifically described with reference to Examples and Comparative Examples. Hereinafter, "parts" are based on weight unless otherwise specified. In addition, tests and evaluations are as follows.
[0168] [Number average molecular weight (Mn) and molecular weight distribution (Mw / Mn)]
[0169] Measured as a polystyrene-equivalent value by gel permeation chromatography (GPC) using tetrahydrofuran as a solvent. In addition, HLC-8320 (manufactured by Tosoh Co.) was used as a measuring instrument, two TSKgel Super Multipore HZ-H (manufactured by Tosoh Co.) were connected in series as a column, and a differential refractometer RI-8320 and an ultraviolet-visible detector (UV-Vis) were used as detectors. The wavelength was set to 254 nm) (manufactured by Tosoh Corporation).
[0170] [Nuclear magnetic resonance spectrum (NMR) measurement]
[0171] First, 30 mg of a polyether compound as a sample was added to 1.0 mL of deuterated di...
manufacture Embodiment A
[0173] (Living Anionic Polymerization of Epichlorohydrin)
[0174] 2.84 g of tetra-n-butylammonium azide and 50 ml of toluene were added to a glass reactor with a stirrer replaced with argon, and the mixture was cooled to 0°C. Next, a solution of 1.256 g of triethylaluminum (1.1 equivalent to tetra-n-butylammonium azide) dissolved in 10 ml of n-hexane was added and reacted for 15 minutes to obtain a catalyst composition. 15.0 g of epichlorohydrin was added to the obtained catalyst composition, and polymerization reaction was performed at 0°C. After the polymerization reaction is initiated, the viscosity of the solution increases slowly. After reacting for 12 hours, a small amount of water was injected into the polymerization reaction solution to terminate the reaction. The obtained polymerization reaction liquid was washed with 0.1N hydrochloric acid aqueous solution to perform deslagging treatment of catalyst residues, and after washing with ion-exchanged water, the organic...
manufacture Embodiment B
[0176] (Coupling of the azido group of polyepichlorohydrin A by click chemistry)
[0177] 1.5 g of polyepichlorohydrin A and 10 ml of dimethylformamide were added to a glass reactor with a stirrer replaced with argon, and kept at room temperature. Next, 0.287 g of copper bromide (I), 0.347 g of pentamethyldiethylenetriamine, and 0.053 g of 1,7-octadiyne were added to the above solution, and reacted at room temperature for 48 hours. The obtained polymerization reaction solution was extracted with toluene, and the organic phase was washed with ion-exchanged water to remove residues of the catalyst, and the organic phase was dried under reduced pressure at 50° C. for 12 hours. The yield of a colorless and transparent oily substance thus obtained was 1.4 g. In addition, the number average molecular weight (Mn) of the obtained substance measured by GPC using a differential refractometer was 2800, and the molecular weight distribution (Mw / Mn) was 1.26. The resulting material was a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com