Tulobuterol crystal form and preparation method thereof
A technology of tulobuterol and crystal, applied in the field of medicinal chemistry synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037](1) Synthesis of α-bromo-2-chlorophenyl (3)
[0038]230.0 g (1.5 mol) of o-chlorophenylideophenone and 1188 ml of water were added to the 3L three-necked bottle, 20 ° C, and 84 ml (1.6 mol) of broshor was added dropwise at 0.5 h, stirred for 15 min. With 400 ml of dichloromethane, the organic layer was washed with a water solution of 4.7 mol / L sodium carbonate, dried over anhydrous sodium sulfate. Filtration, evaporation of the solvent to evaporated, 346.3 g of light yellow oily liquid, yield 99%. ESI-MS M / Z: 234.0 [M + H]+139.1 [M-CH2BR]+111.1 [m-coch2BR]+.
[0039](2) Synthesis of 1- (2-chlorophenyl) -2-bromide ethanol (4)
[0040]347.4 g (1.5 mol) 3 was dissolved in 1126.9 ml of anhydrous ethanol, and 42.48 g (0.74 mol) of potassium borohydride was added dropwise under ice bath conditions, and the temperature of 244.6 ml was prepared, and the control temperature was below 20 ° C, drop Bi, continue to stir 15 min. The ethanol was evaporated under reduced pressure, and 760 mL of w...
Embodiment 2
[0054]50 g of Totorro was added to 100 mL of acetone, heated to 40 to 50 ° C to stir the dissolution. After dissolving, the acetone solution of Totorro was cooled to 30 to 40 ° C, and 300 ml of n-hexane was slowly added dropwise with the dropping rate of 4 ml / min, and the temperature was held at 30 to 40 ° C. The mixture was stirred for 8 hours or more, filtered crystals, and extract filtered cakes for 12 hours to give 43.2 grams, yield; 86.4%, HPLC purity 99.9%.
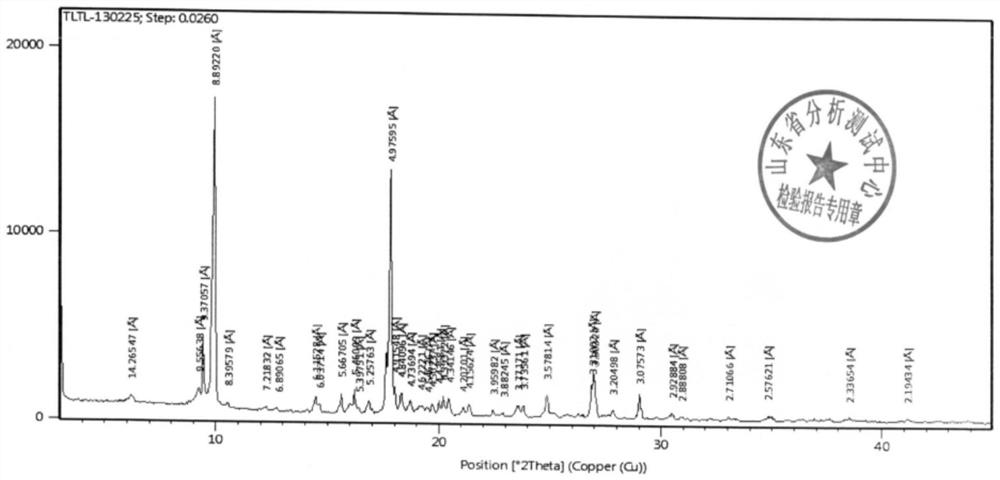
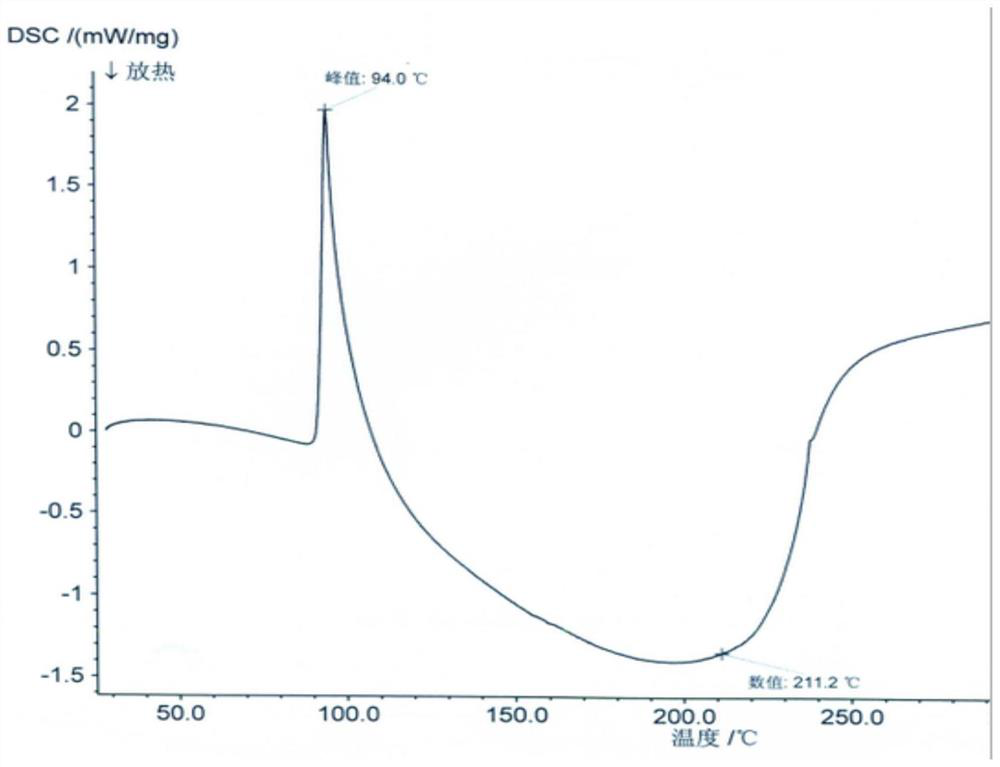
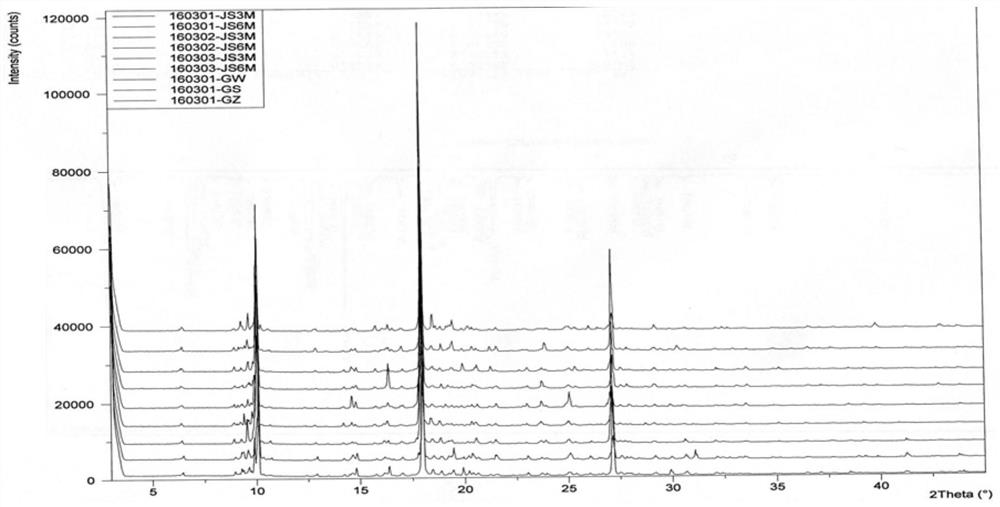
[0055]The XRD and structural confirmation results obtained by the product obtained in this embodiment were the same as in Example 1, and will not be described again.
Embodiment 3
[0057]50 g of Totoro was added to 200 ml of acetonitrile, heated to 40 to 50 ° C, stirred and dissolved. After dissolving, the acetone solution of the Totoro was cooled to 30 to 40 ° C, and 800 ml of cyclohexane was slowly added dropwise with a dropwise increase of 4 ml / min, and the temperature was held at 30 to 40 ° C. The mixture was stirred for 8 hours or more, filtered crystals, and dried into vacuum for 12 hours to obtain 47.2 grams of product, yield; 94.4%, HPLC purity 99.8%.
[0058]The XRD and structural confirmation results obtained by the product obtained in this embodiment were the same as in Example 1, and will not be described again.
PUM
| Property | Measurement | Unit |
|---|---|---|
| strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com