Kit for detecting ABL tyrosine kinase inhibitor based on HPLC-MS/MS method and detection method
A technology of tyrosine kinases and inhibitors, applied in the field of compositions, can solve problems such as ineffective treatment of patients, achieve the effects of improving detection quality, promoting standardization and standardization, and improving accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
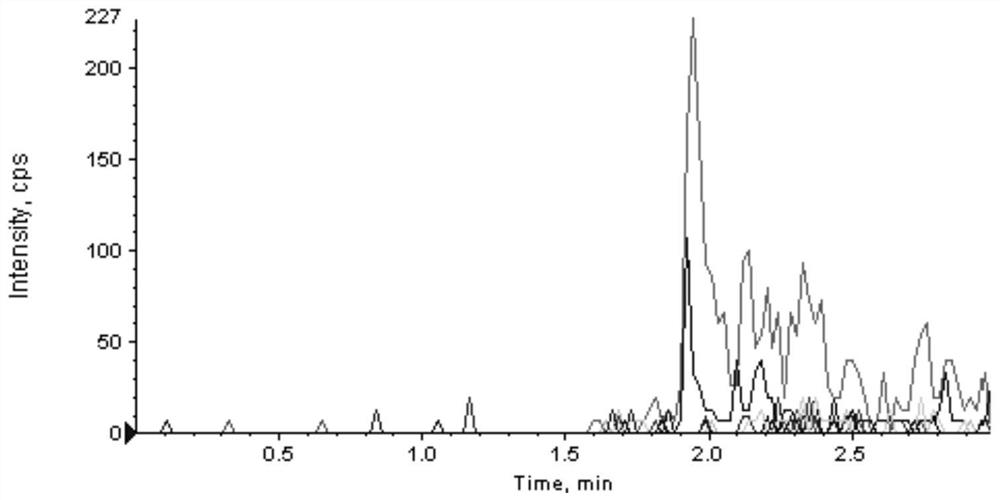
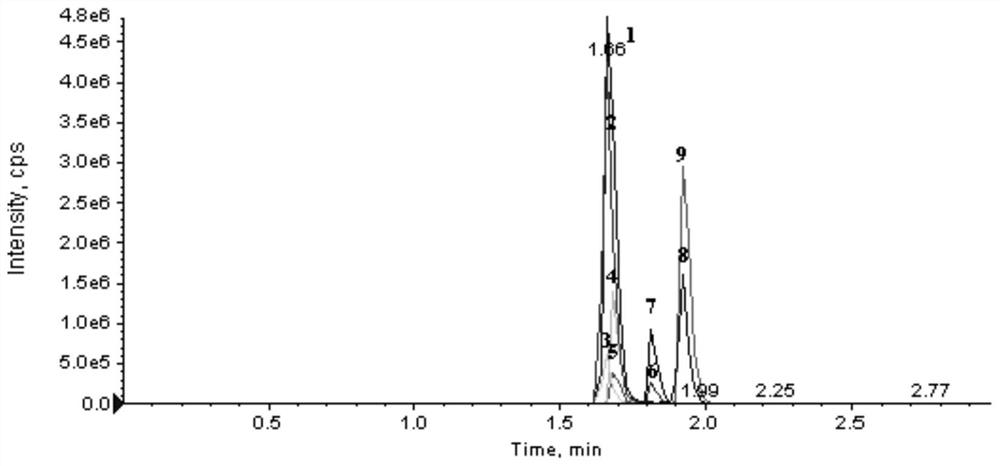
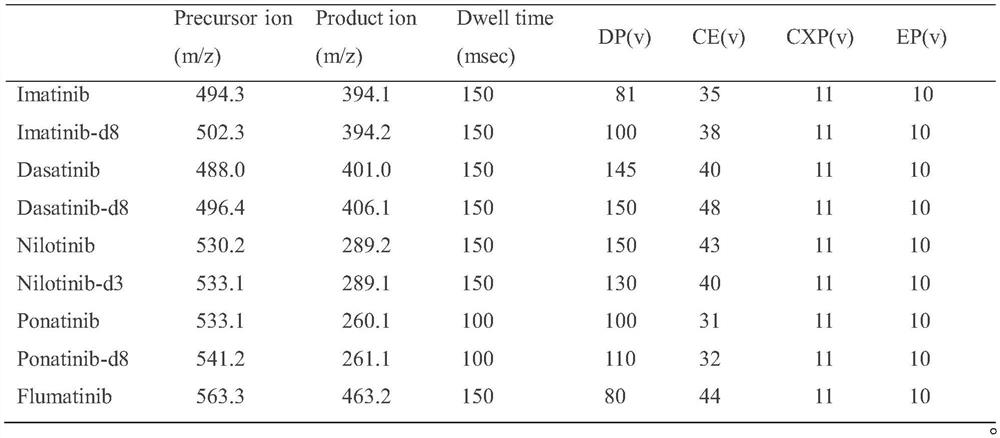
[0045] This embodiment is a kit for detecting ABL tyrosine kinase inhibitors based on the HPLC-MS / MS method. ABL tyrosine kinase inhibitors include imatinib, dasatinib, nilotinib, pana tinib and flumatinib;
[0046] The kit includes the following reagents:
[0047] Sample processing solution, standard mother solution, quality control product, internal standard solution and mobile phase, the mobile phase includes mobile phase A and mobile phase B
[0048] Mobile phase A is 0.1% formic acid methanol solution;
[0049] Mobile phase B is 2mmol·L -1 Ammonium acetate-0.1% formic acid aqueous solution;
[0050] The sample processing solution is 2mmol L -1 Ammonium acetate-0.1% formic acid aqueous solution;
[0051] Standard stock solutions include 100 μg / mL Imatinib methanol solution, 100 μg / mL Nilotinib methanol solution, 10 μg / mL Dasatinib methanol solution, 10 μg / mL Ponatinib methanol solution and 10 μg / mL Flumatinib methanol solution;
[0052] Quality control products inclu...
Embodiment 2
[0058] The present embodiment is the preparation method of the kit in Example 1, and the preparation method comprises the following steps:
[0059] 1. Preparation of standard products:
[0060] Preparation of standard product mother solution: 100 μg / mL Imatinib methanol solution Preparation: 1 mg Imatinib standard product, dissolved in methanol, and fixed to a 10 mL volumetric flask with methanol, ultrasonically mixed, dispensed for use; the same operation as above to obtain 100 μg / mL Nilotinib methanol solution;
[0061] 10μg / mL Dasatinib methanol solution preparation: 1mg Dasatinib standard, dissolved in methanol, and dilute to a 10mL volumetric flask with methanol, ultrasonically mixed, dispensed for use, that is, 100μg / mL Dasatinib, take 100μL 100μg / mL Dasatinib stock solution, Add 900 μL of methanol, shake and mix to obtain a 10 μg / mL Dasatinib methanol solution; operate as above to obtain a 10 μg / mL Ponatinib methanol solution and 10 μg / mL Flumatinib methanol solution; ...
Embodiment 3
[0087] This embodiment is a method for detecting ABL tyrosine kinase inhibitors using the above kit, the method comprising:
[0088] Take 100 μL of the sample to be tested and add 2 mmol·L -1 Ammonium acetate-0.1% formic acid aqueous solution 100μL, then add 300μL of internal standard solution containing ABL tyrosine kinase inhibitor, vortex and mix for 30-60S, high-speed centrifugation (13200rpm) for 8min, pipette 170μL of supernatant, put it on the machine for testing, Perform HPLC-MS / MS quantitative analysis;
[0089] Wherein, the high performance liquid chromatography conditions are as follows:
[0090] Using Ultimate XB-C18 chromatographic column (4.6×50mm, 5um), column temperature 60°C; flow rate 0.8mL min -1 ; Mobile phase A is 0.1% formic acid methanol solution, mobile phase B is 2mmol L -1 Ammonium acetate-0.1% formic acid aqueous solution, injection volume 5uL;
[0091] The elution method is gradient elution, and the gradient elution procedure is as follows:
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com