Preparation method of 2-methoxyethylamine
A technology of methoxyethylamine and methoxyethyl, which is applied in the field of synthesis of 2-methoxyethylamine, can solve problems such as difficult industrial production, cumbersome reaction routes, and equipment corrosion, and achieve simple operation and preparation The effect of simple route and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: the preparation of 2-methoxyethylamine
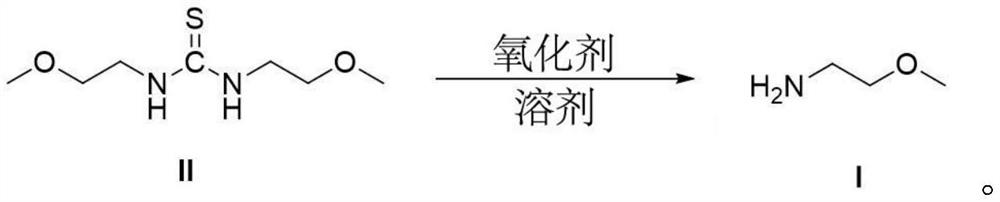
[0030] In a 250ml round bottom flask, add N,N'-bis(2-methoxyethyl)thiourea (19.2g, 0.1mol), 40ml of acetonitrile, 120ml of water, potassium persulfate (81g, 0.3mol), Heat to reflux at 80°C for 6h (GC tracks the reaction progress); the reaction formula is as follows:
[0031]
[0032] After the reaction was finished, acetonitrile was distilled off; cooled to room temperature, added 100ml of pure water, extracted with dichloromethane (40ml*3), separated into layers, the organic phase was dried over anhydrous sodium sulfate and concentrated to obtain a crude product; purified by distillation (collecting 87 -90°C distillate) to obtain 12.525 g of pure product with a yield of 83.5%.
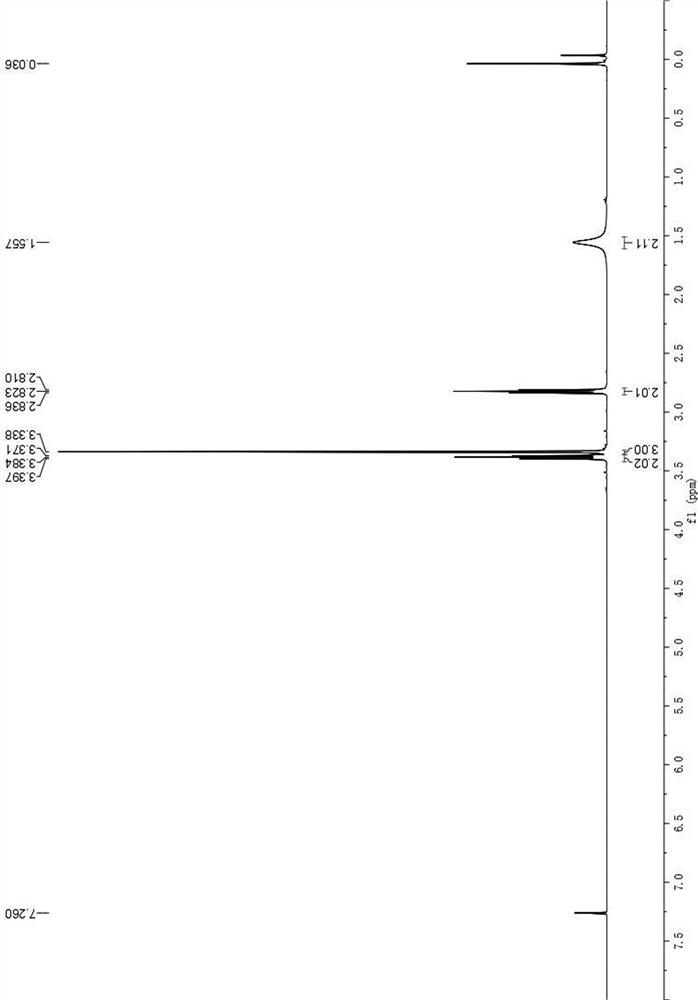
[0033] The H NMR and C NMR data of the product are as follows: 1 H NMR (400MHz, CDCl 3 )δ3.38(t, J=5.2Hz, 2H), 3.34(s, 3H), 2.82(t, J=5.2Hz, 2H), 1.56(s, 2H).
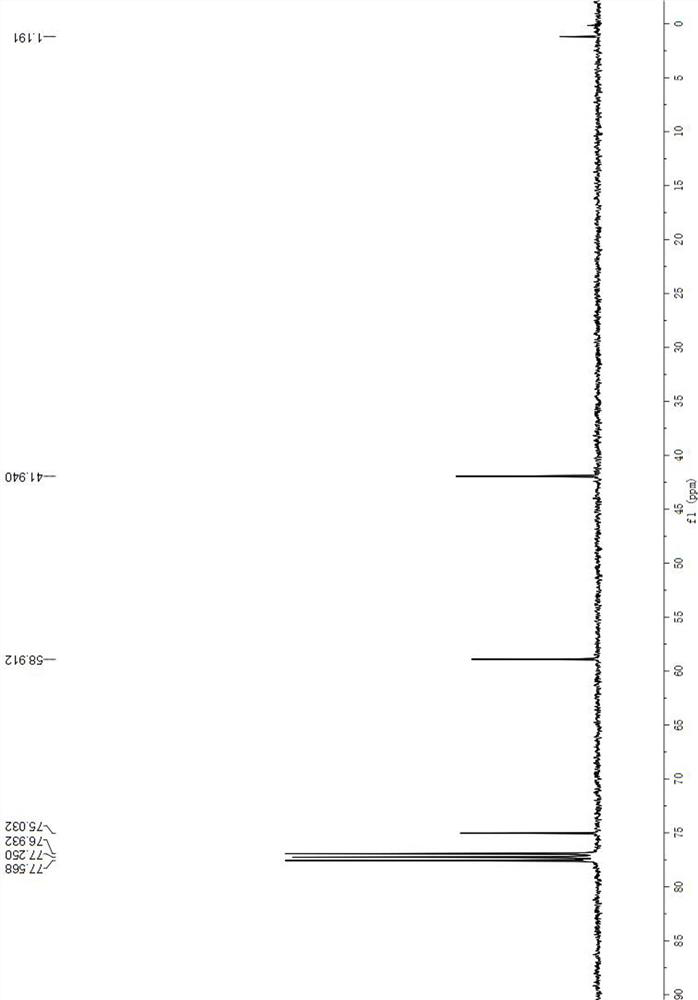
[0034] 13 C NMR (100MHz, CDCl 3 )δ77.03,58.91,41.94.
[0035] The str...
Embodiment 2
[0037] Embodiment 2: the screening of oxidizing agent
[0038] Experimental conditions of the present embodiment, charging amount are identical with embodiment 1, select different oxidant (3 times (molar ratio) of raw material II) to carry out experiment, specifically as shown in table 1:
[0039] Table 1
[0040] oxidizing agent yield 1 tert-butanol peroxide 46.2% 2 hydrogen peroxide 13.5% 3 potassium persulfate 83.5% 4 sodium persulfate 78.3%
[0041] As can be seen from Table 1, when hydrogen peroxide is selected as oxidant, the reaction yield is the lowest, only 13.5%. Is 83.5%; In summary, the present invention preferably potassium persulfate as reaction oxidant.
Embodiment 3
[0042] Embodiment 3: potassium persulfate (K 2 S 2 o 8 ) dosage screening
[0043] The experimental conditions of the present embodiment, the feeding amount are identical with embodiment 1, select different doses of K 2 S 2 o 8 Carry out the experiment, specifically as shown in Table 2:
[0044] Table 2
[0045] Dosage (mol) yield 1 0.1 58.5% 2 0.2 71.3% 3 0.3 83.5% 4 0.4 84.0%
[0046] As seen from Table 2, when the consumption of potassium persulfate was 0.1mol, the reaction yield was only 58.5%; when the consumption was 0.3mol, the reaction yield was 83.5%. Efficiency does not obviously improve; In sum, the present invention selects 0.3mol potassium persulfate optimally.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com