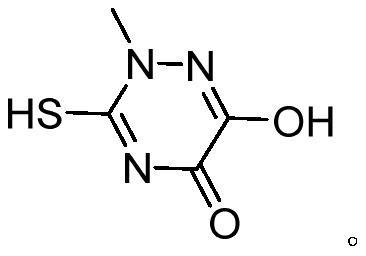
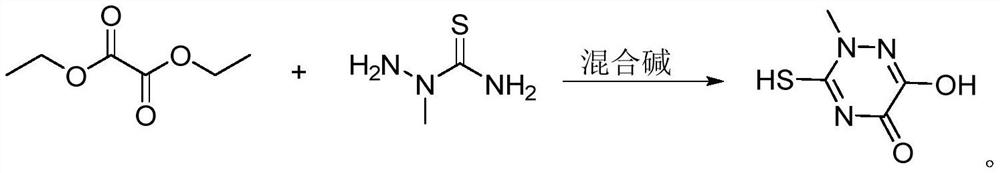
The preparation method of triazine ring
A technology of triazine ring and methylthiosemicarbazide, applied in the direction of organic chemistry, can solve the problems of complex process, inconvenient operation, high cost, etc., and achieve the effect of cheap and easy-to-obtain raw materials, avoid difficult recycling, and easy recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 2-Methylaminothiourea 10.5g, diethyl oxalate 15.3g, methanol 31.5g, ethanol 31.5g were added to the reaction bottle, reduced to 15 °C, and the mixed alkali catalyst (including sodium methanol 36.8g, DMAP 0.05g, TMEDA 0.05g) was added dropwise, 1.5h, Drop-plus Bi temperature to 55 °C for cyclic reaction 2.5h, hydrochloric acidification to pH = 1.0, cooling to 5 °C filtration, to obtain triazine ring crude product, crude product 70 °C dissolved in distilled water 63g, cooling to 15 °C crystallization, filtration, to obtain triazine ring wet product, 100 °C dry 3h, to obtain triazine ring dry product 14.75g, purity 99.6%, yield 92.7%.
Embodiment 2
[0038]The reaction bottle was added 2-methylthiourea 10.5g, diethyl oxalate 16.1g, methanol 15.8g, ethanol 36.8g, reduced to 5 °C, dropwise mixed alkali catalyst (including sodium methanol 26.3g, DMAP 0.01g, TMEDA 0.01g) 0.5h, drops plus Bi heating to 45 °C for cyclic reaction for 4h, Hydrochloric acidification to pH = 0.5, cooling to 0 °C filtration, to obtain triazine ring crude product, crude product 65 °C dissolved in distilled water 52.5g, cooled to 10 °C crystallization, extraction filtration, to obtain triazine ring wet product, 90 °C drying for 5h, to obtain dried triazine ring 14.66g, purity 99.7%, yield 92.1%.
Embodiment 3
[0040] The reaction bottle was added 2-methylthiourea 10.5g, diethyl oxalate 15.6g, methanol 24g, ethanol 36g, reduced to 10 °C, dropwise mixed alkali catalyst (including sodium methanol 30g, DMAP 0.02g, TMEDA 0.03g) 1h, dropwise added Bi heating to 50 °C for cyclic reaction 3h, Hydrochloric acidification to pH = 0.7, cooling to 3 °C filtration, to obtain triazine ring crude product, crude product 68 °C dissolved in distilled water 60g, cooled to 13 °C crystallization, filtration, to obtain triazine ring wet product, 95 °C dry 4h, to give triazine ring dry product 14.72g, purity 99.6%, yield 92.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com