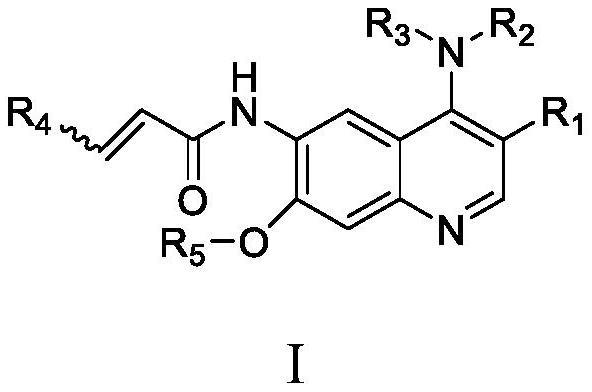
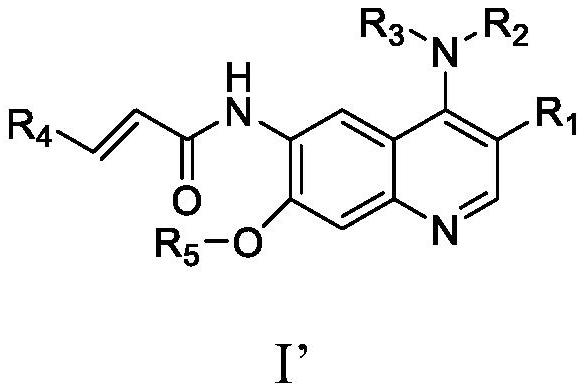
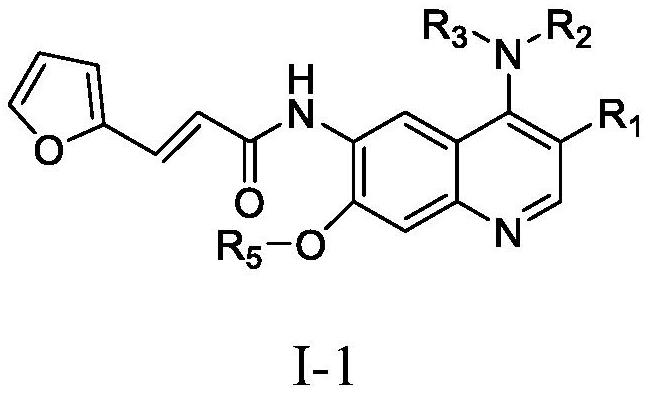
Quinoline derivative containing furyl and preparation method and application thereof
A technology of alkyl group and group is applied in the field of quinoline derivatives containing furanyl group and preparation thereof, and can solve the problems of high non-selectivity, acute toxicity or cell drug resistance of antitumor drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0098] Example 1: (E)-N-(4-(4-(benzyloxy)anilino)-3-cyano-7-ethoxyquinolin-6-yl)-3-(furan-2- base) the synthesis of acrylamide (a)
[0099]
[0100] (1) Synthesis of trans-3-(2-furyl)acryloyl chloride (a-1)
[0101] Add trans-3-(2-furyl)acrylic acid into a 50mL three-necked flask equipped with a thermometer and electromagnetic stirring
[0102] (0.003mol, 0.41g), 7mL thionyl chloride, reflux reaction under nitrogen protection for 4h, the remaining solvent was evaporated under reduced pressure, and the solvent was removed under reduced pressure to obtain 0.44g of light yellow oil with a yield of 95.0%.
[0103] (2) Synthesis of N-(4-(4-(benzyloxy)anilino)-3-cyano-7-ethoxyquinolin-6-yl)acetamide (a-2)
[0104] Add N-(4-chloro-3-cyano-7-ethoxyquinolin-6-yl)acetamide (0.024mol, 6.90g) into a 500mL three-necked flask equipped with a thermometer and electromagnetic stirring, 4- (Benzyloxy) aniline (0.026mol, 5.17g) and (0.026mol, 3.0g) pyridine hydrochloride, 250mL isopropanol, ...
Embodiment 2
[0114] Example 2: (E)-N-(4-(4-chloroanilino)-3-cyano-7-ethoxyquinolin-6-yl)-3-(furan-2-yl)acrylamide Synthesis of (b)
[0115]
[0116] (1) Synthesis of N-(4-(4-chloroanilino)-3-cyano-7-ethoxyquinolin-6-yl)acetamide (b-1)
[0117] According to the method of step (2) in Example 1, add N-(4-chloro-3-cyano-7-ethoxyquinolin-6-yl) in a 500mL three-necked flask equipped with a thermometer and electromagnetic stirring Acetamide (0.024mol, 6.90g), 4-chloroaniline (0.026mol, 3.30g), and (0.026mol, 3.00g) pyridine hydrochloride, 250mL isopropanol, TLC tracking detection, developing solvent is petroleum ether: The volume ratio of ethyl acetate was 1:1, stirred and refluxed for 12h, allowed to stand, filtered with suction, and recrystallized from methanol to obtain intermediate b-1 (7.90g, 87%).
[0118] 1 H NMR (DMSO-d 6 ):δ11.09(br,s,1H),9.62(s,1H),9.08(s,1H),9.01(s,1H),7.62(s,1H),7.53-7.56(m,2H), 7.44-7.47(m,2H),4.33(q,J=6.8Hz,2H),2.11(s,3H),1.50(t,J=6.8Hz,3H)
[0119] (2) Syn...
Embodiment 3
[0126] Example 3: (E)-N-(4-(4-(3-fluorobenzyloxy)-3-chloroaniline)-3-cyano-7-ethoxyquinolin-6-yl)-3 Synthesis of -(furan-2-yl)acrylamide (c)
[0127]
[0128](1) N-(4-(4-(3-fluorobenzyloxy)-3-chloroaniline)-3-cyano-7-ethoxyquinolin-6-yl)acetamide (c-1) Synthesis
[0129] According to the method of step (2) in Example 1, add N-(4-chloro-3-cyano-7-ethoxyquinolin-6-yl) in a 500mL three-necked flask equipped with a thermometer and electromagnetic stirring Acetamide (0.024mol, 6.90g), 4-(3-fluorobenzyloxy)-3-chloroaniline (0.026mol, 6.53g) and (0.026mol, 3.0g) pyridine hydrochloride, 250mL isopropanol, TLC tracking detection, the developer is a mixture of petroleum ether: ethyl acetate with a volume ratio of 1:1, stirred and refluxed for 12 hours, allowed to stand, filtered with suction, and recrystallized from methanol to obtain intermediate c-1 (10.52g, 87%).
[0130] 1 H NMR (DMSO-d 6 ):δ11.09(br,s,1H),9.62(s,1H),8.98-9.10(m,2H),7.59-7.63(m,2H),7.27-7.50(m,5H),7.17-7.21(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com