High-stereoselectivity methionine adenosyltransferase as well as preparation method and application thereof
A methionine adenosine, stereoselective technology, applied in the field of protein and enzyme engineering, can solve problems such as no reports, and achieve the effects of convenient operation, reduced production cost and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
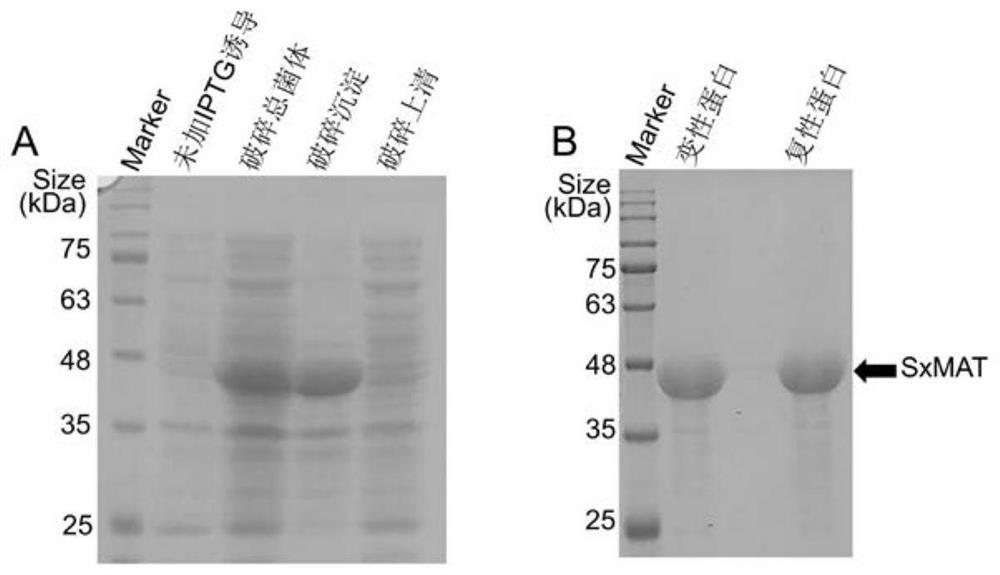
[0048] Embodiment 1: the preparation of a kind of highly stereoselective methionine adenylyltransferase (SxMAT)
[0049] 1. Experimental method
[0050] Specific steps are as follows:
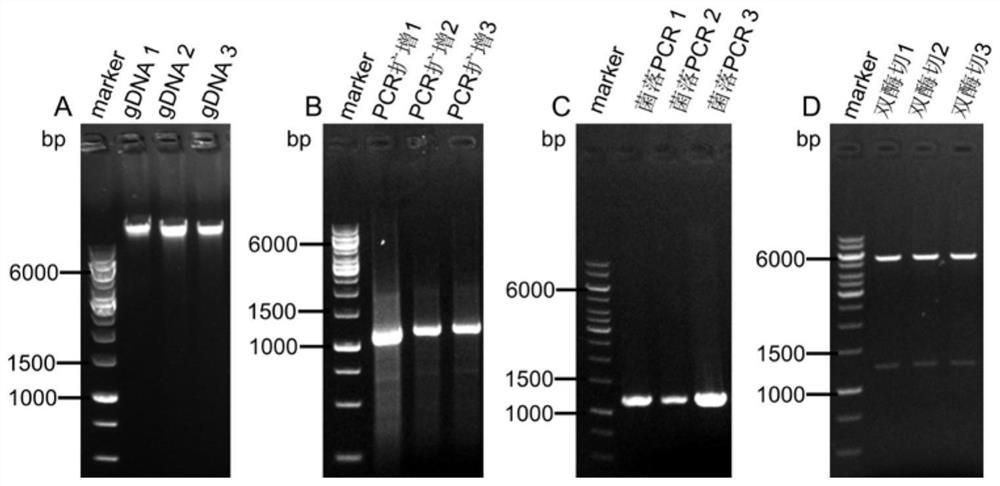
[0051] 1) extracting the genome of Streptomyces xinghai;
[0052] After culturing Streptomyces xinghai in potato medium for 5-8 days, use a genome extraction kit to extract the genome (gDNA) of Streptomyces xinghai, dilute it 10 times and store it at -20°C for later use;
[0053] 2) using the genome in step 1) as a DNA template, using PCR amplification technology to obtain the gene sequence of the methionine adenylyltransferase in Streptomyces xinghai, which is the metK target fragment;
[0054] Design upstream and downstream primers containing NdeI and XhoI restriction sites respectively, use the genome (gDNA) extracted in step 1) as a DNA template, perform PCR amplification according to Table 1 and Table 2, to obtain the purpose of the MAT gene sequence metK fragment.
[0055] Table 1 The...
Embodiment 2
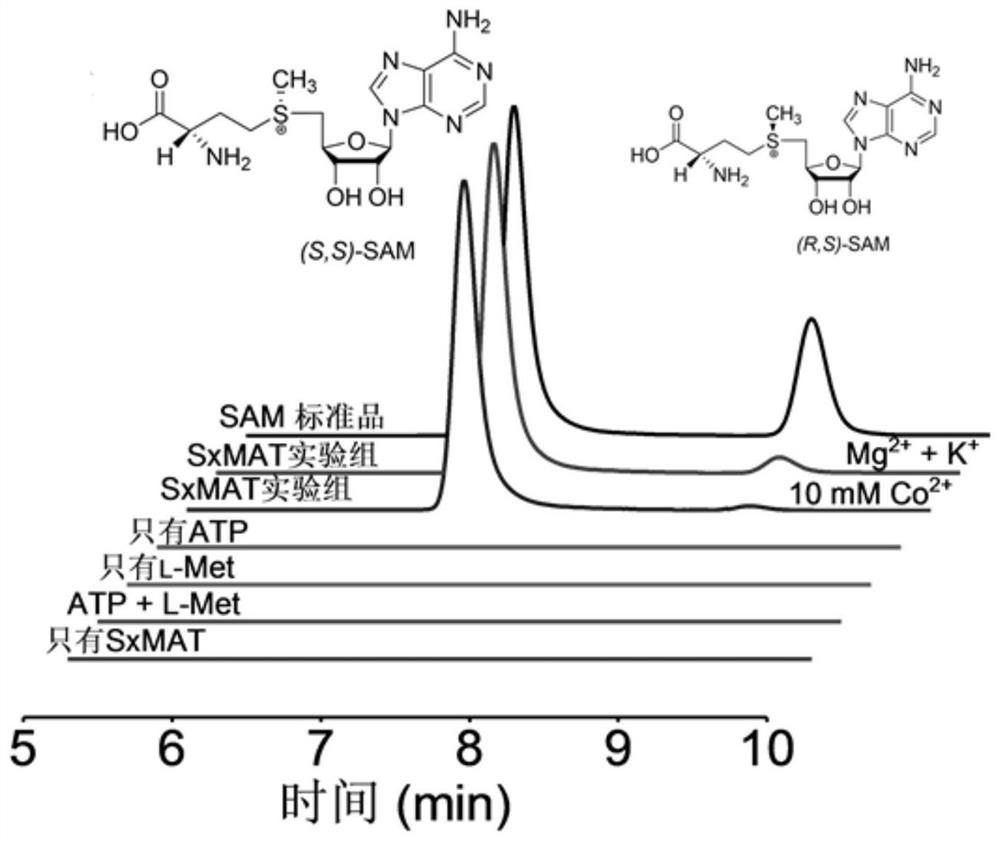
[0072] Embodiment 2: HPLC and LC-MS detect the biological activity of SxMAT
[0073] 1. Experimental materials
[0074] SxMAT, Tris-HCl buffer solution, L-Met, ATP, metal ion K prepared by the method in Example 1 + -Mg 2+ / Co 2+ 、TCA
[0075] 2. Experimental method
[0076] The reaction was carried out in 100 mM Tris-HCl buffer, pH 8.5, in a total volume of 500 μL, which contained SxMAT (6 mg / mL), substrates L-Met (5 mM) and ATP (5 mM), metal ion K + (150mM)-Mg 2+ (20mM) / Co 2+ (10mM), at the same time set up negative control groups containing only ATP, only L-Met, only SxMAT, only ATP and L-Met. TCA was inactivated, centrifuged at 12000 rpm / min for 10 min, and the supernatant was filtered through a 0.22 μm filter membrane for HPLC and LC-MS analysis.
[0077] 3. Experimental results
[0078] by such as image 3 It can be seen that the results show that only the experimental group has product peaks, while the negative control group has no product peaks. Figure 4 It ...
Embodiment 3
[0079] Example 3: Determination of the influence of temperature on the activity of SxMAT and the thermal stability of SxMAT
[0080] 1. Experimental materials
[0081] SxMAT, Tris-HCl buffer solution, L-Met, ATP, metal ion K prepared by the method in Example 1 + -Mg 2+ 、TCA
[0082] 2. Experimental method
[0083] Determination of the optimum reaction temperature of SxMAT: The reaction was carried out in 100mM, pH 8.5 Tris-HCl buffer in a total volume of 500μL, which contained SxMAT (6mg / mL), substrate L-Met (5mM) and ATP ( 5mM), metal ion K + (150mM)-Mg2 + (20mM), each reacted at 130rpm / min in a shaker at 25°C, 35°C, 45°C, 55°C, 65°C, 75°C, and 85°C for 2h, and then added 150μL of 12% TCA was inactivated, centrifuged at 12000rpm / min for 10min, and the supernatant was taken to pass through the membrane for HPLC analysis.
[0084] Determination of SxMAT thermal stability: The reaction was carried out in 100mM Tris-HCl buffer, pH 8.5, with a total volume of 500μL, which con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com