General low-cost quaternary ammonium salt sugar chain isotope labeling reagent and synthesis method
A technology of isotope labeling and quaternary ammonium salts, applied in the field of biological glycome analysis, can solve the problems of poor economical use, difficulty in wide application, and expensive synthetic raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
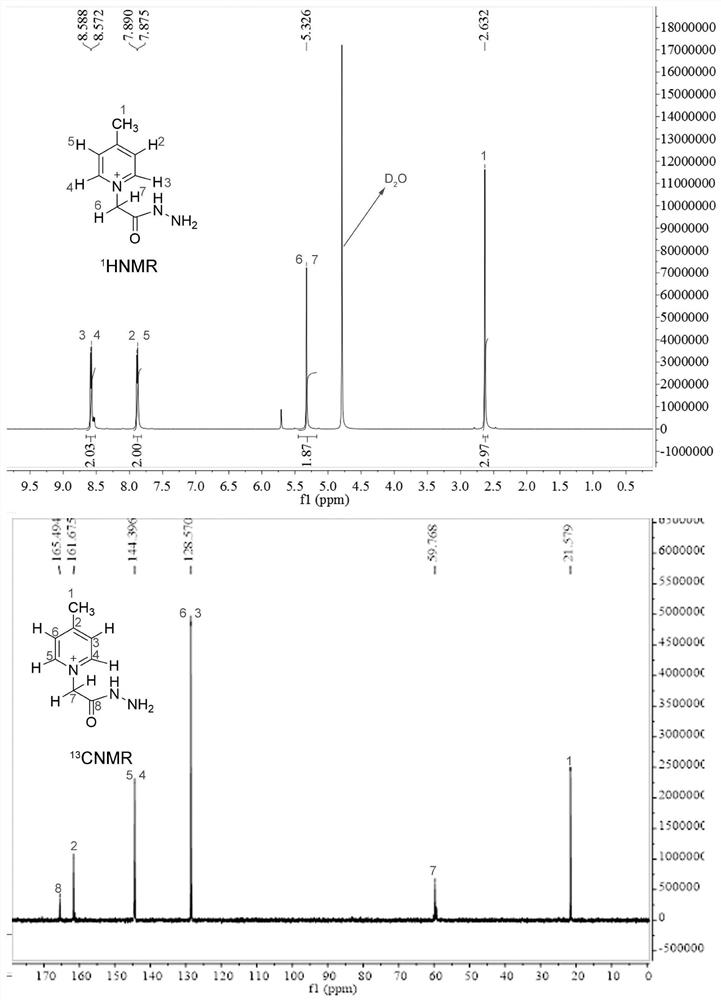
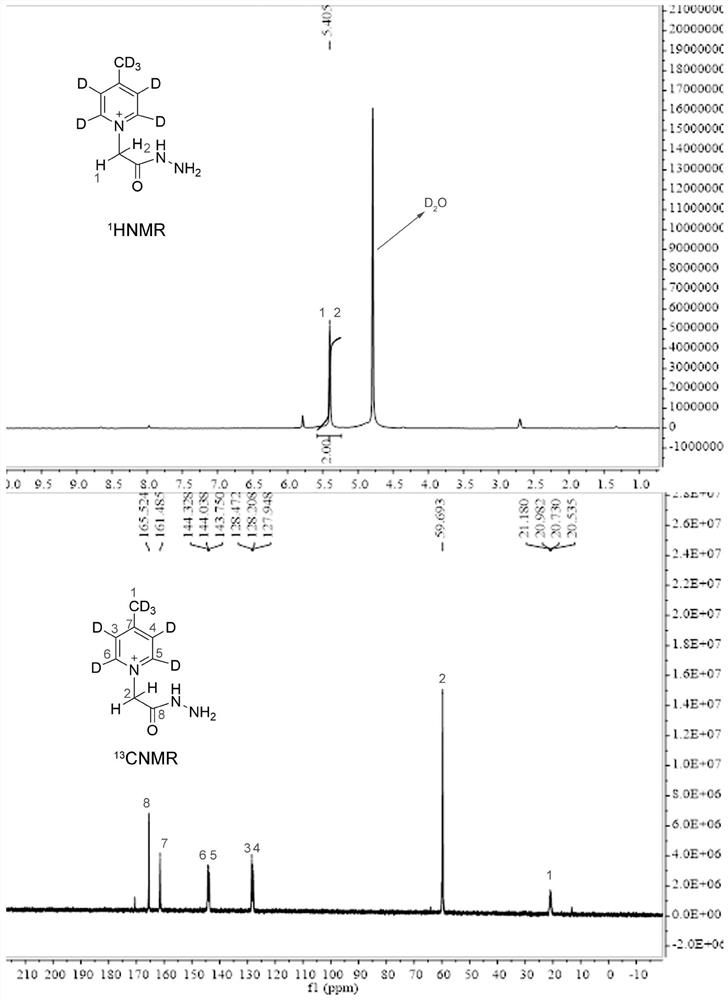
[0058] A method for synthesizing a universal low-cost quaternary ammonium salt sugar chain isotope labeling reagent, the specific steps are as follows:
[0059] S1. Place a 25 mL round bottom flask containing 10 mL of methanol on an electronic balance, weigh 1.0 g of deuterated 4-picoline into the round bottom flask, and then weigh 2.0 g of ethyl bromoacetate into the round bottom flask. Build a condensation reflux device, stir and reflux at 70°C for 12 hours;
[0060] S2, place the obtained mixture in an ice bath, add 1.2 mL of 80% hydrazine hydrate dropwise under stirring, control the flow rate to complete the dropwise addition within 30 minutes, then seal the round bottom flask, and continue to stir and react in the ice bath for 2 hours;
[0061] S3. After the reaction is completed, use a rotary evaporator to concentrate the reaction solution to an oily state, then add 10mL of absolute ethanol that has been pre-cooled at -20°C in advance, stir in an ice bath for 30 minutes,...
Embodiment 2
[0065] A general low-cost quaternary ammonium salt-based sugar chain isotope labeling reagent for mass spectrometry detection sensitivity evaluation of sugar chain derivatives, the specific operation steps are as follows:
[0066] S1, weigh 9.9mg of maltohexaose and 11.34mg of β-cyclodextrin, and prepare 1mmol / L aqueous solution respectively, take the solutions of maltohexaose and β-cyclodextrin, mix them at a molar ratio of 5:1, and obtain a mixture solution Analyzed by MALDI-TOF MS for later use.
[0067] S2, weigh 24.5 mg of HMP and dissolve it in 1 mL of solvent (methanol: water: glacial acetic acid = 6:3:1) to prepare a 0.1M HMP reaction solution.
[0068]The mixture samples in S3 and S1 were concentrated and dried by centrifugation, added 25 uL of HMP reaction solution, reacted in a 70°C water bath for 1 h, concentrated by centrifugation to remove the solvent, and then dissolved in 1 mL of ultrapure water for MALDI-TOF MS detection.
Embodiment 3
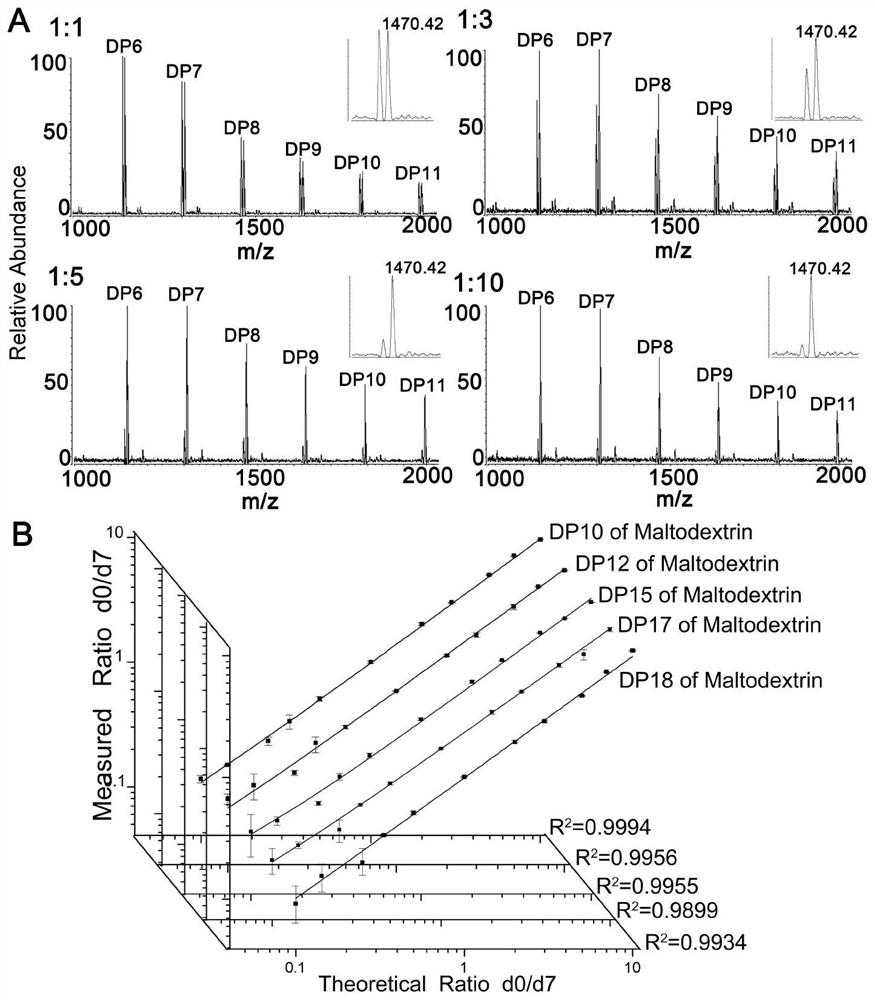
[0076] Quantitative linear range and quantitative stability investigation of a universal low-cost quaternary ammonium salt-based sugar chain isotope labeling reagent. The sugar chain standard used is maltodextrin. The specific steps are as follows:
[0077] S1, weigh 1 mg of maltodextrin and dissolve it in 0.5 mL of ultrapure water to prepare a sugar chain standard solution.
[0078] S2, weigh 24.5mg HMP and dissolve it in 1mL solvent (methanol: water: glacial acetic acid = 6:3:1) to prepare 0.1M d 0 -HMP reaction solution; weigh 31.5mg d 7 -HMP was dissolved in 1mL solvent (methanol: water: glacial acetic acid = 6:3:1) to prepare 0.1M d 7 - HMP reaction solution.
[0079] S3, use a micro-sampling needle to take 1uL sugar chain standard solution and add it to a 0.5mL centrifuge tube, prepare 2 parts in total, and then add 25uLd 0 -HMP reaction solution and d 7 - HMP reaction liquid, reacted in a water bath at 70°C for 1 h, then centrifuged, concentrated and dried to remove...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com