1h-indazole-3-carboxylic acid derivative and preparation method of glasstron and lonidamine
A technology of carboxylic acid derivatives and indazole, which is applied in the field of chemistry, can solve the problems of difficult preparation of raw materials, low total yield, and long synthetic route, and achieve cheap and easy-to-obtain raw materials and reagents, short route, and short reaction time Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0059] The preparation method of 1H-indazole-3-carboxylic acid derivative;
[0060] Method 1: The present invention uses anthranilamide or anthranilate as a starting material, and then synthesizes and prepares 1H-indazole-3-carboxylic acid derivatives in the next step under the action of sodium nitrite or tert-butyl nitrite. The reaction conditions are mild, the reaction time is short, the yield is high, and the raw materials and reagents used are cheap and easy to obtain. The specific steps are as follows:
[0061] S1. Anthranilamide derivatives or anthranilyl acetate compounds, tert-butyl nitrite, glacial acetic acid, and organic solvents are prepared in a molar equivalent of 1:1:1:5~1:3:4:20 Proportionally added to the reaction kettle, stirred at room temperature for 0.5 to 8 hours;
[0062] S2. After the reaction is completed, add water equal to the volume of the organic solvent and ethyl acetate equal to the volume of the organic solvent for extraction, wash the organic ...
Embodiment 1
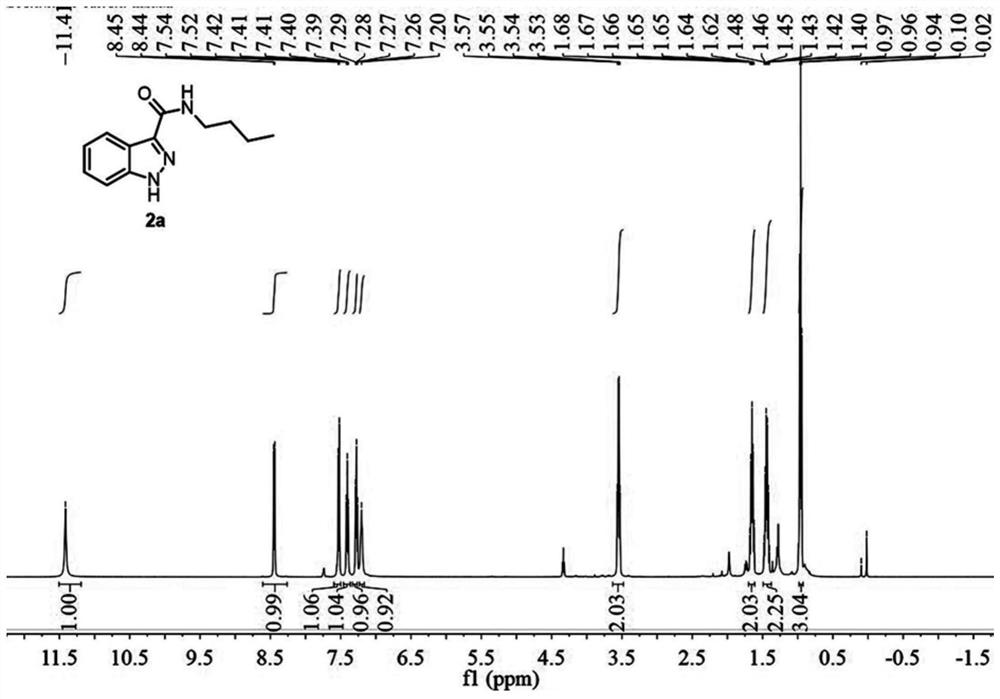
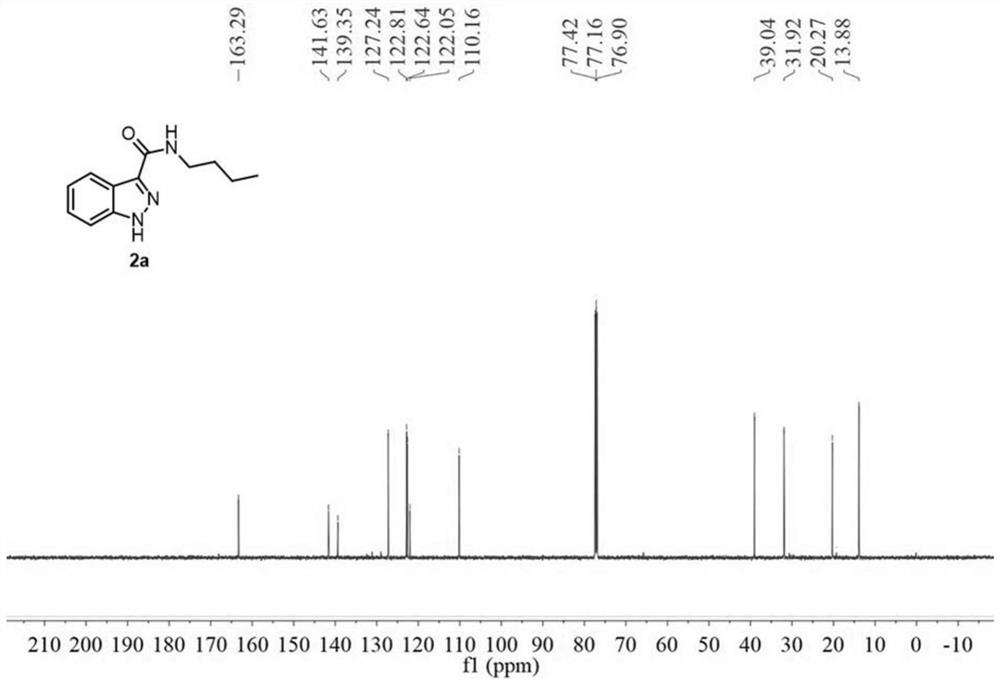
[0073] A preparation method of 1H-indazole-3-carboxylic acid derivative (2a), specifically as follows:
[0074] 2a was prepared as follows:
[0075]
[0076] Weigh 34.8mg (0.2mmol) of N-n-butyl-2-aminophenylacetamide (1a), dissolve it in 2mL of acetonitrile (analytical grade), and place it in a 10mL reaction kettle at room temperature 20°C-25°C Stir, then add 23uL (0.4mmol, 2.0equiv) glacial acetic acid, and two minutes later add 36uL (0.3mmol, 1.5equiv) tert-butyl nitrite. During the reaction, the solution first turned light yellow, and then an orange solid precipitated out. When the orange solid disappeared and the solution turned into an orange clear solution, the reaction was complete, and the reaction time was 1 hour. After the reaction was completed, 10.0 mL of water and 10.0 mL of ethyl acetate were added for extraction, and the organic phase was washed with saturated sodium carbonate solution and saturated brine respectively, then dried with anhydrous sodium sulfat...
Embodiment 2
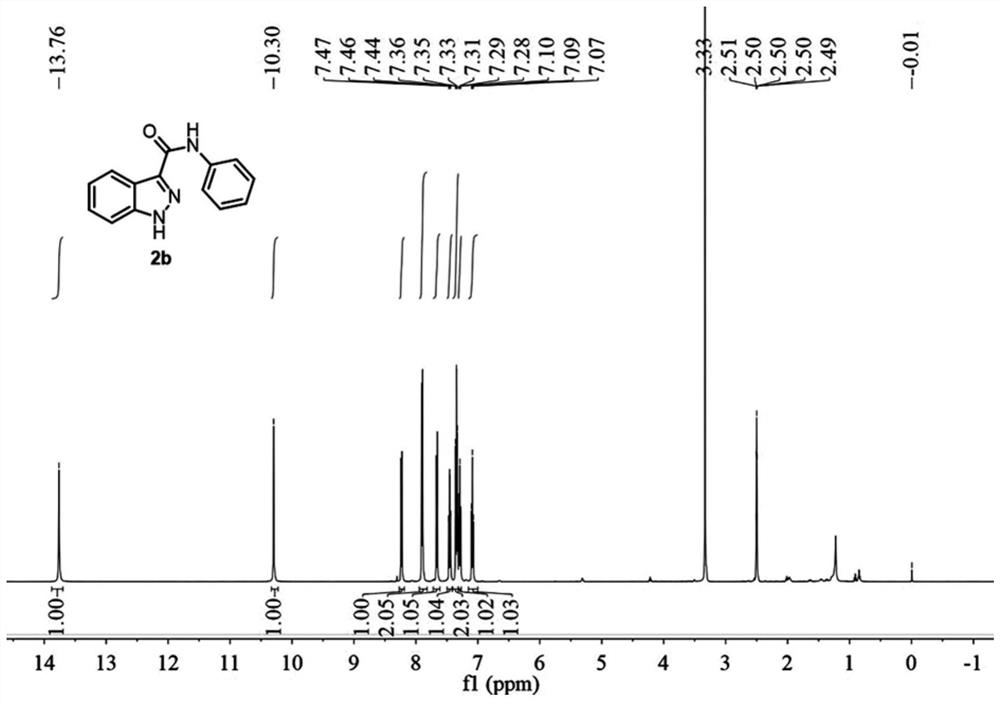
[0079] A preparation method of 1H-indazole-3-carboxylic acid derivative (2b), specifically as follows:
[0080] The preparation method of 2b is the same as that of Example 1, and the raw material is 2-(2-aminophenyl)-N-phenylacetamide.
[0081]
[0082] The reaction time was 3 hours, and the yield was 89%.
[0083] Compound 2b is a known compound, CAS: 23706-99-2. Such as image 3 and Figure 4 as shown, 1 H NMR (500MHz, DMSO-d 6 )δ13.76(s,1H),10.30(s,1H),8.29–8.09(m,1H),8.08–7.78(m,2H),7.76–7.62(m,1H),7.45(t,J= 7.5Hz, 1H), 7.38–7.23(m, 2H), 7.09(t, J=7.5Hz, 1H). 13 C NMR (125MHz, DMSO-d 6 )δ160.96, 141.30, 138.87, 138.31, 128.56, 126.71, 123.39, 122.34, 121.76, 121.53, 120.21, 110.86. HRMS (ESI) calcd for C 14 h 12 ON 3 + ([M+H] + ):238.0975, Found: 238.0976.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com