A kind of organic non-fullerene electron acceptor material and its preparation method and application
An electron acceptor material, non-fullerene technology, applied in the fields of organic chemistry, electro-solid devices, semiconductor/solid-state device manufacturing, etc., can solve the problems of low electron mobility, device performance to be improved, insufficient chemical stability, etc. Achieve the effect of high electron mobility, improved electron transport capability, strong visible light or near-infrared light absorption performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
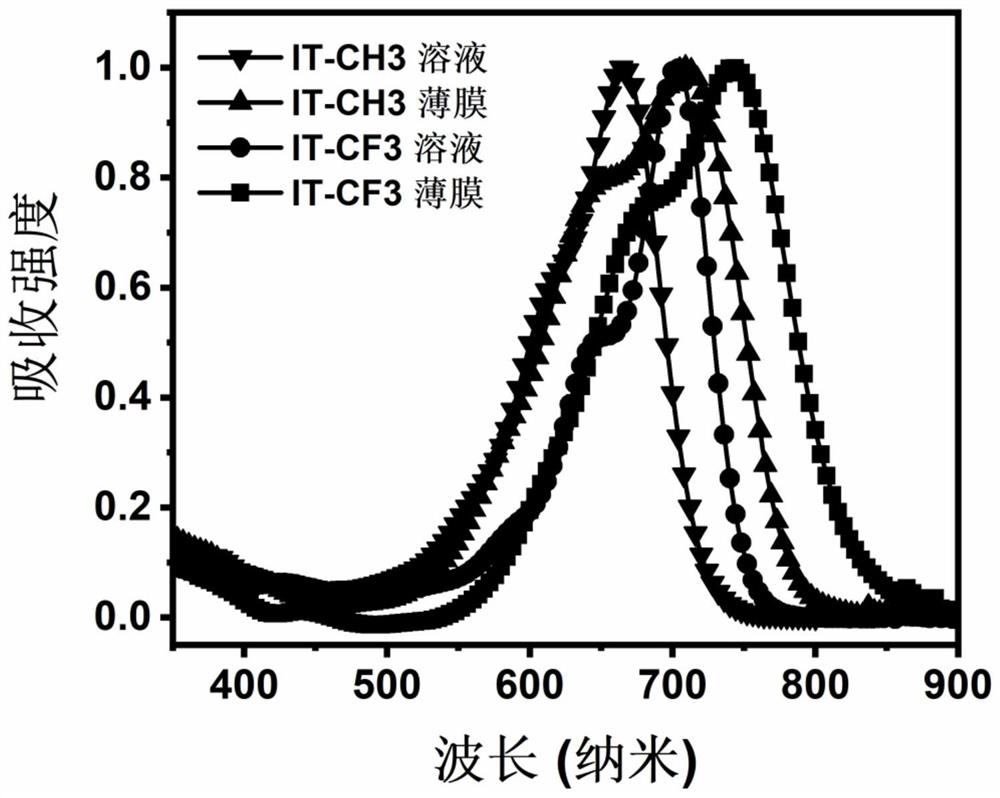
[0046] The condensed ring non-fullerene acceptor material (IT-CF3) structural formula of this embodiment is:
[0047]
[0048] The preparation method of IT-CF3 comprises the following steps (1) to (4):
[0049] (1) Synthesis of 4-trifluoromethylphthalic anhydride
[0050]
[0051] Under the protection of nitrogen, add 4-trifluoromethylphthalic acid (2g) and acetyl chloride (15mL) into a 100mL three-necked flask, heat to reflux for 12 hours, remove the acetyl chloride with a rotary evaporator, and dry it with an oil pump to obtain a white solid. Further processing, directly used as the next step of synthesis.
[0052] (2) Synthesis of 6-trifluoromethyl-1,3-indandione
[0053]
[0054] Under a nitrogen atmosphere, mix 4-trifluoromethylphthalic anhydride (1g, 5mmol), acetic anhydride (10mL) and triethylamine (5mL), and slowly add tert-butyl acetoacetate (0.8g , 6mmol). Stir overnight at room temperature, pour concentrated hydrochloric acid and ice water after the rea...
Embodiment 2
[0068] The condensed ring non-fullerene acceptor material (YCF3) structural formula of this embodiment is:
[0069]
[0070] The preparation method of YCF3 comprises following steps (1)~(4):
[0071] (1) to (3) are the same as in Example 1.
[0072] (4) Synthesis of YCF3
[0073]
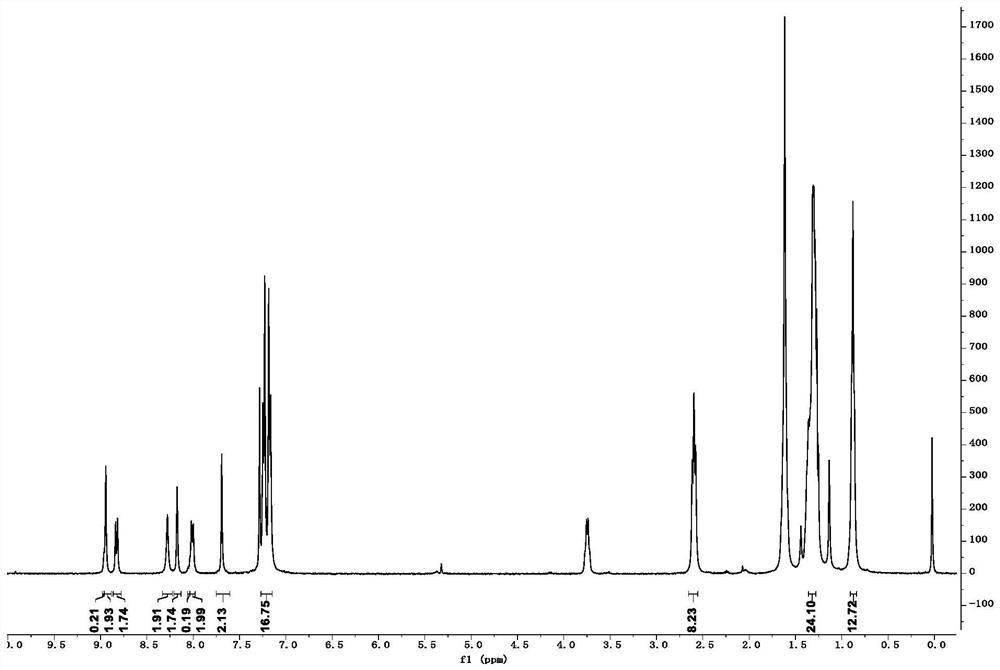
[0074] Add Y6-CHO (100mg, 0.1mmol), trifluoromethylcyanindanone (70mg, 0.3mmol) and anhydrous chloroform (15mL) obtained in step (3) to a 25mL flask, and react at 65 degrees Celsius under nitrogen protection for 12 hours . After the reaction, return to room temperature, add water to wash three times, and saturated brine to wash three times, then dry the organic phase with anhydrous sodium sulfate, and separate the product by column chromatography to obtain the product as black solid YCF3 (50 mg). The H NMR spectrum is: 1 H NMR (400MHz, CDCl 3 ):δ8.97(s,0.5H),8.94(s,1.5H),8.84(d,1.5H),8.28(s,2H),8.17(s,2H),8.04(s,0.5H), 8.00(d,2H),7.69(s,2H),7.28(d,8H),7.18(d,8H),2.60(t,8H),1.66(t,8H),1.3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electron mobility | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com