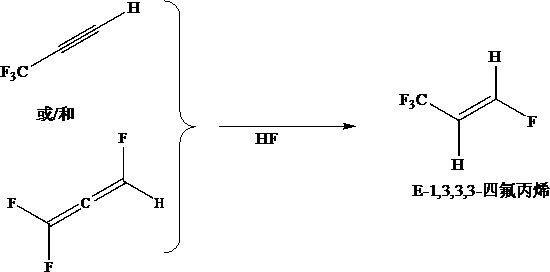
Preparation method of E-1, 3, 3, 3-tetrafluoropropene
A technology of tetrafluoropropene and 1.E-1, which is applied in the field of preparation of E-1,3,3,3-tetrafluoropropene, can solve the problems of low single-pass yield, high reaction energy consumption, and low reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
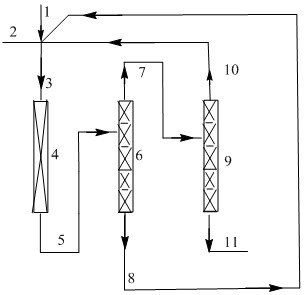
Image
Examples
Embodiment 1
[0042] The preparation of embodiment 1 trifluoropropadiene
[0043] Step (1): Dissolve an appropriate amount of silver nitrate in an appropriate amount of water according to the mass percentage of silver oxide and carrier, add aluminum fluoride carrier, impregnate for 18 hours, then filter, dry in an oven at 80°C for 24 hours, and then place Under the protection of nitrogen, calcined at 400° C. for 10 hours to obtain the oxidation catalyst. Fill 10 milliliters of the oxidation catalyst 20%Ag prepared by the above-mentioned method in the tubular reactor made of Incon alloy with an inner diameter of 1 / 2 inch and a length of 30 cm 2 O / AlF 3 . The reaction conditions are as follows: the reaction temperature is 260°C, the molar ratio of oxygen to 1,2,3,3,4,4,5-heptafluorocyclopentene is 1:1, the contact time is 0.25s, and the reaction pressure is 0.1MPa. The reaction product was collected by a polytetrafluoroethylene sampling bag. After 10 hours, a sample was taken from the samp...
Embodiment 2
[0045] Example 2 Preparation of 3,3,3-trifluoropropyne
[0046] Preparation of isomerization catalyst: Put molybdenum trioxide in the reactor, at a temperature of 400°C, pass a mixed gas composed of chlorodifluoromethane and nitrogen with a substance ratio of 1:4, activate for 12 hours, stop the flow into the mixed gas to obtain the catalyst.
[0047] 10 mL of the above-prepared catalyst was filled in a tubular reactor made of Inconium alloy with an inner diameter of 1 / 2 inch and a length of 30 cm. The reaction conditions are as follows: the reaction temperature is 20° C., the contact time of trifluoropropadiene is 60 s, and the reaction pressure is 0.1 MPa. After running for 10 hours, the reaction product was collected and heated, and the gas phase organic phase was taken for GC analysis. The reaction results are: the conversion rate of trifluoropropadiene is 100%, and the selectivity of 3,3,3-trifluoropropyne is 99.8%.
Embodiment 3
[0050] Preparation of fluorination catalyst: According to the percentage composition of tungsten and cobalt elements of 90% and 10%, tungsten trichloride and cobalt nitrate are dissolved in water, concentrated ammonia water is added dropwise for precipitation, the pH value is adjusted to 7.5, and then aged 24 hours, washed with water, filtered, dried in an oven at 120°C for 15 hours, crushed the obtained solid, and pressed into tablets to obtain a catalyst precursor. Put 10 mL of the catalyst precursor into a Monel with an inner diameter of 1 / 2 inch and a length of 30 cm Tubular reactor made of material, filled with nitrogen and baked at 350°C for 12 hours, nitrogen space velocity is 200h -1 , and then lower the temperature to 300°C, and at the same time pass a mixed gas composed of hydrogen fluoride and nitrogen with a mass ratio of 1:2, and the total space velocity of the gas is 220h -1 , activate for 12 hours, stop the above mixed gas, and prepare the tungsten-based catalys...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com